A38002
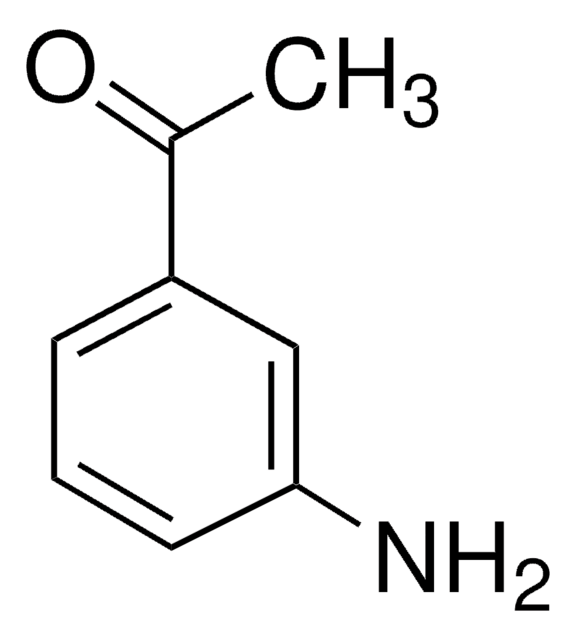
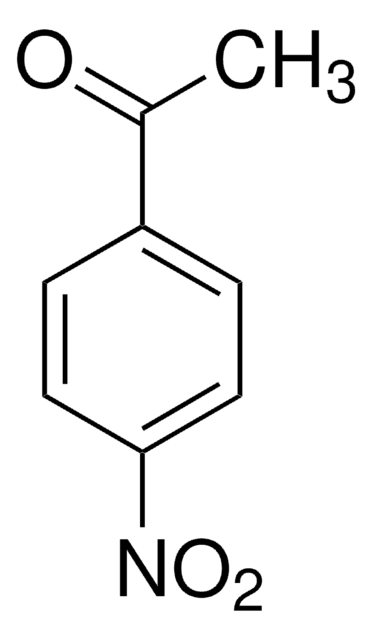
4′-Aminoacetophenone
99%
Sinónimos:
4-Acetylaniline
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
H2NC6H4COCH3
Número de CAS:
Peso molecular:
135.16
Beilstein/REAXYS Number:
471493
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
99%
form
powder
bp
293 °C (lit.)
mp
103-107 °C (lit.)
SMILES string
CC(=O)c1ccc(N)cc1
InChI
1S/C8H9NO/c1-6(10)7-2-4-8(9)5-3-7/h2-5H,9H2,1H3
InChI key
GPRYKVSEZCQIHD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Anne Ly et al.
Organic & biomolecular chemistry, 3(5), 917-923 (2005-02-26)
The most easily oxidized sites in DNA are the guanine bases, and major intermediates produced by the direct effect of ionizing radiation (ionization of the DNA itself) are electron deficient guanine species. By means of a radiation chemical method (gamma-irradiation
Dominik Cincić et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 4), o226-o229 (2008-04-09)
In the title compounds, 4-carboxyanilinium bromide, C(7)H(8)NO(2)(+) x Br(-), (I), and 4-acetylanilinium bromide, C(8)H(10)NO(+) x Br(-), (II), each asymmetric unit contains a discrete cation with a protonated amino group and a halide anion. Both crystal structures are characterized by two-dimensional
O A Aleksintseva et al.
Antibiotiki, 27(7), 493-495 (1982-01-01)
The effect of p-aminobenzoic acid on the biosynthesis of levorin was studied. It was shown that in the presence of exogenic p-aminobenzoic acid the antibiotic activity increased by 11 per cent. The acid added was transformed into p-aminoacetophenone which was
O A Aleksintseva et al.
Antibiotiki, 26(8), 566-570 (1981-08-01)
A method for spectrophotometric determination of p-aminoacetophenone (p-AAP) in the mycelium and fermentation broth filtrates of organisms producing polyenic macrolide antibiotics is described. The level of p-AAP accumulation was studied as applicable to the biosynthesis of levorin, a polyenic antibiotic
O Raatikainen et al.
Journal of chromatography, 585(2), 247-254 (1991-11-01)
A high-performance liquid chromatographic (HPLC) method for the determination of the aromaticity of heptaene polyene antibiotics has been developed. The released aromatic moiety of the heptaene polyenes aureofungin, candicidin, candimycin, hamycin and trichomycin was assayed after alkaline hydrolysis. The presence
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico