930962
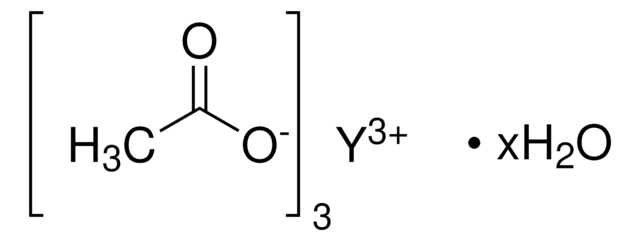
Yttrium(III) acetate tetrahydrate
99.99% trace rare earth metals basis
Sinónimos:
Acetic acid yttrium(3+) salt, Yttrium triacetate
About This Item
Productos recomendados
Quality Level
assay
99.99% trace rare earth metals basis
form
powder
impurities
≤150 ppm trace rare earth metals
≤500 ppm trace metals
mp
350 °C (Decomp.)
solubility
H2O: soluble (lit.)
density
1.5 g/cm3
Categorías relacionadas
General description
Application
A major application of high-purity yttrium acetate is in the synthesis of sodium yttrium fluoride (NaYF4) nanoparticles. Typically, in these syntheses, yttrium acetate is mixed with oleic acid in octadecene and heated to form Y(oleate)3, which is reacted with ammonium fluoride and sodium hydroxide in methanol at modest temperatures (e.g. 50 C) to form NaYF4 nanoparticles. This synthesis offers great control over particle size and crystallinity and allows for easy incorporation rare-earth metal dopants.
Lanthanide-doped NaYF4 nanoparticles are one of the most studied materials for up conversion. These nanoparticles, which can convert two photons of near-infrared (NIR) light into visible light, have important in-vivo applications because of the deep tissue penetration abilities of NIR. For example, these nanoparticles have been used for in-vivo Zn2+ optical sensing, in-vivo ratiometric sensing of lymphatic inflammation,, and in-vivo sensing of peroxynitrite.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico