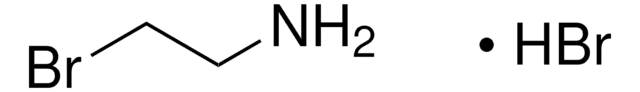
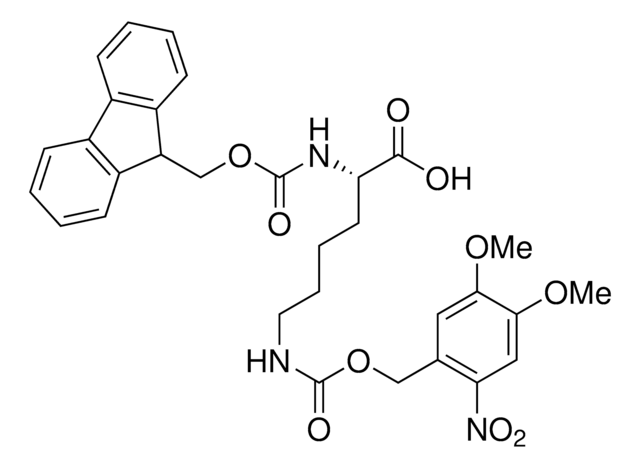
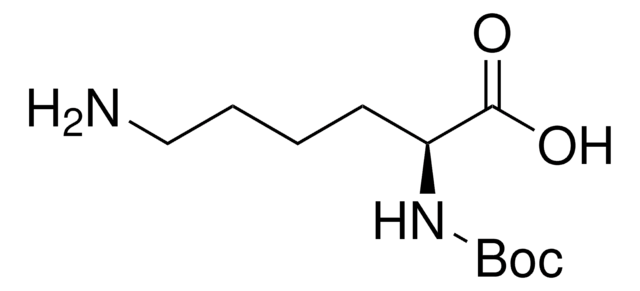
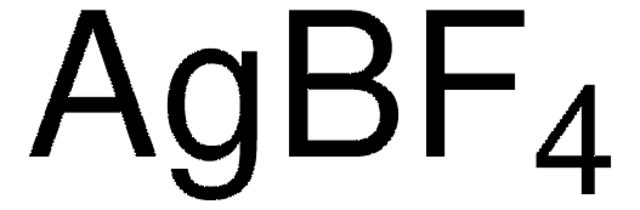913588
O-(2-Nitrobenzyl)-L-tyrosine hydrochloride
≥95%
Sinónimos:
(S)-2-Amino-3-(4-((2-nitrobenzyl)oxy)phenyl)propanoic acid hydrochloride, NBY, ONBY, Photo-controlled amino acid, Photocaged amino acid, Photocleavable tyrosine derivative
About This Item
Productos recomendados
assay
≥95%
form
powder
availability
available only in USA
mp
205 °C (decomp.)
storage temp.
2-8°C
InChI
1S/C16H16N2O5.ClH/c17-14(16(19)20)9-11-5-7-13(8-6-11)23-10-12-3-1-2-4-15(12)18(21)22;/h1-8,14H,9-10,17H2,(H,19,20);1H/t14-;/m0./s1
InChI key
DRUCEARMIBXBOJ-UQKRIMTDSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Time-resolved protein activation by proximal decaging in living systems
Crystal structure of a domain-swapped photoactivatable sfGFP variant provides evidence for GFP folding pathway
Light-control of the ultra-fast Gp41-1 split intein with preserved stability of a genetically encoded photo-caged amino acid in bacterial cells
Rapid and Inexpensive Evaluation of Nonstandard Amino Acid Incorporation in Escherichia coli
Related product
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![N-[2-(Fmoc-amino)-ethyl]-Gly-O-tBu hydrochloride ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)

![[2,2′-Bipyridine]-6-carboxylic acid hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/130/786/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d/640/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d.png)