913294
keYPhos™
Umicore
Sinónimos:
CyYPhos(Me)PCy2, Tricyclohexyl(1-(dicyclohexyl-phosphanyl)ethylidene)-phosphane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C32H58P2
Número de CAS:
Peso molecular:
504.75
UNSPSC Code:
12352001
NACRES:
NA.22
Productos recomendados
product name
keYPhos™,
form
powder
Quality Level
reaction suitability
reagent type: ligand
mp
167-169 °C
functional group
phosphine
General description
keYPhos™is an ylide-functionalized phosphine ligand developed in the lab of Prof. V. Gessner at the Ruhr-University Bochum with demonstrated uses in Pd-catalyzed cross coupling reactions, including the arylation of ketones and arylation of amines. keYPhos™ is part of the YPhos™ family of ligands, also containing the joYPhos™ and trYPhos™ ligands.
Application
The electron-rich and sterically demanding keYPhos™ has a methyl group in the ylide-backbone and is a valuable ligand for the palladium catalyzed coupling of aryl chlorides with primary and secondary alkyl and aryl amines at room temperature. keYPhos™has been used in the gold(I)-catalyzed hydroamination of acetylene, and has shown to be effective in a range of Buchwald-Hartwig amination reactions. The strong electron-donor strength and sterically demanding nature of the ligand has been shown to increase the rate of formation of the catalytically active mono-phosphine palladium species, often leading to decreased reaction times or allowing the use of lower reaction temperatures.
Learn more about ylide-functionalized phosphines (YPhos)
Learn more about ylide-functionalized phosphines (YPhos)
Features and Benefits
Advantages of the keYPhos™ligand over less electron rich ligand sources include, increased substrate scope in Buchwald-Hartwig amination reactions, including aryl chlorides, the use of more mild reaction conditions and improved activity in in C-N and C-C cross coupling reactions. keYPhos™ has been shown to perform well with common palladium sources such as Pd2(dba)3, Pd(OAc)2, [Pd(allyl)Cl]2 or [Pd(cinamyl)Cl]2.
Legal Information
Product of Umicore
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
Yphos is a trademark of Umicore AG & Co. KG
Related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Jens Tappen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(19), 4281-4288 (2020-01-24)
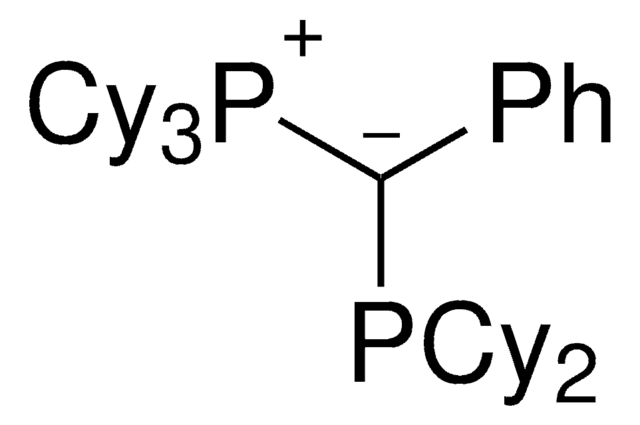
Palladium allyl, cinnamyl, and indenyl complexes with the ylide-substituted phosphines Cy3 P+ -C- (R)PCy2 (with R=Me (L1) or Ph (L2)) and Cy3 P+ -C- (Me)PtBu2 (L3) were prepared and applied as defined precatalysts in C-N coupling reactions. The complexes are
Xiao-Qiang Hu et al.
Organic letters, 21(18), 7558-7562 (2019-08-31)
Ylide-functionalized phosphine (YPhos) ligands allow the palladium-catalyzed α-arylation of alkyl ketones with aryl chlorides with record setting activity. Using a cyclohexyl-substituted YPhos ligand, a wide range of challenging ketone substrates was efficiently and selectively monoarylated under mild conditions. A newly
Ilja Rodstein et al.
The Journal of organic chemistry, 85(22), 14674-14683 (2020-09-11)
Ylide-substituted phosphines have been shown to be excellent ligands for C-N coupling reactions under mild reaction conditions. Here we report studies on the impact of the steric demand of the substituent in the ylide-backbone on the catalytic activity. Two new
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico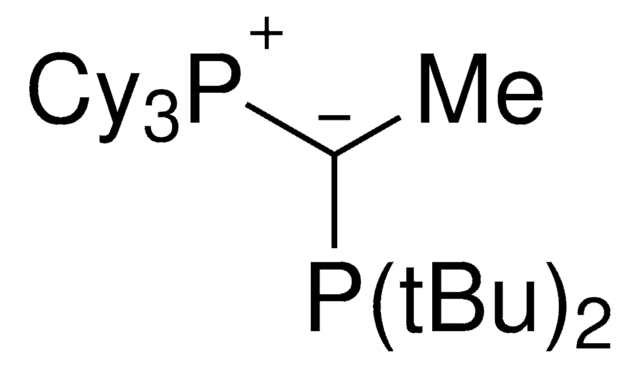
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)


![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)