900258
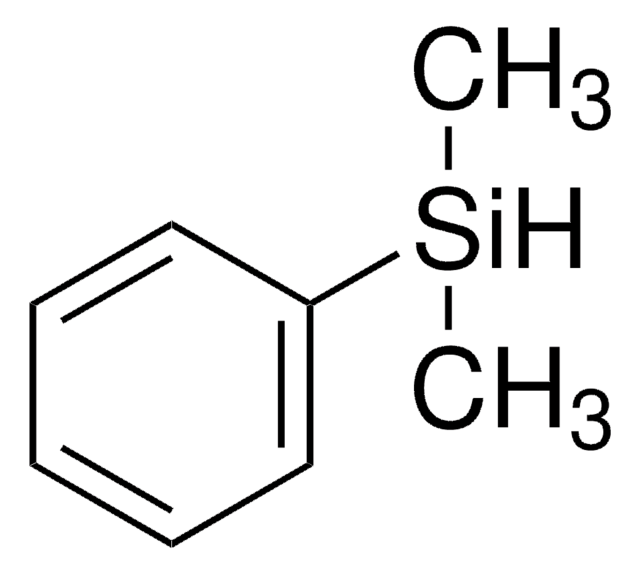
Isopropoxy(phenyl)silane
Sinónimos:
RubenSilane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H14OSi
Número de CAS:
Peso molecular:
166.29
MDL number:
UNSPSC Code:
12352005
NACRES:
NA.22
Productos recomendados
form
liquid
Quality Level
reaction suitability
reagent type: reductant
reaction type: Reductions
refractive index
n/D 1.4799
density
0.926 g/mL
storage temp.
2-8°C
SMILES string
[SiH2](OC(C)C)c1ccccc1
InChI
1S/C9H14OSi/c1-8(2)10-11-9-6-4-3-5-7-9/h3-8H,11H2,1-2H3
InChI key
XYMAHMLQCLMTDN-UHFFFAOYSA-N
General description
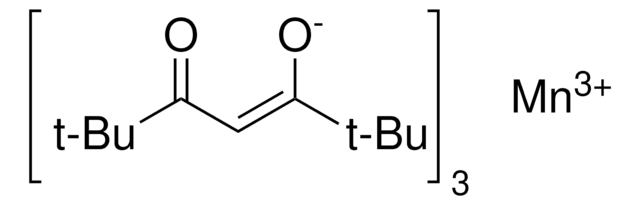
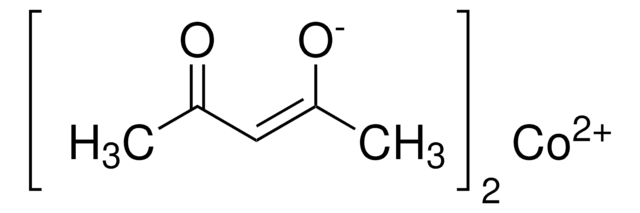
Isopropoxy(phenyl)silane is used as a stoichiometric reductant that allows for a significant decrease in catalyst loading, lower reaction temperatures, a wide range of functional group tolerance, and more diverse solvents in metal-catalyzed Mukaiyama hydrofunctionalizations.
Application
Isopropoxy(phenyl)silane can be used as a hydride source for alkene hydrofunctionalization reactions in the presence of metal catalysts, particularly under aprotic, nonalcoholic conditions. This reagent is considered as one of the most efficient stoichiometric reductants than phenylsilane because of its ability to exclude alcohol solvent from a series of catalytic reactions. When utilized, it allows the researcher to significantly decrease catalyst loadings, lower reaction temperatures, and employ more diverse solvents in iron- and manganese-catalyzed Mukaiyama hydrofunctionalizations.
It can be used as a reagent in:
It can be used as a reagent in:
- The hydrogenation of olefins
- Branch-selective olefin cross-coupling reactions
- Markovnikov alkene hydration/amination reactions
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Carla Obradors et al.
Journal of the American Chemical Society, 138(14), 4962-4971 (2016-03-18)
We report the discovery of an outstanding reductant for metal-catalyzed radical hydrofunctionalization reactions. Observations of unexpected silane solvolysis distributions in the HAT-initiated hydrogenation of alkenes reveal that phenylsilane is not the kinetically preferred reductant in many of these transformations. Instead
Isopropoxy (phenyl) silane
Demoret RM and Shenvi RA
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico