857297
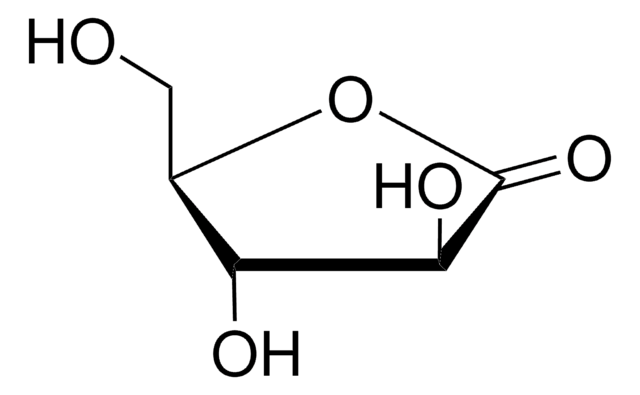
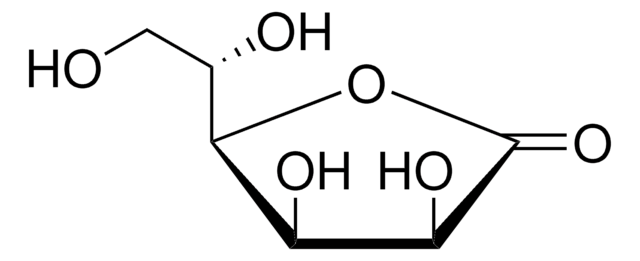
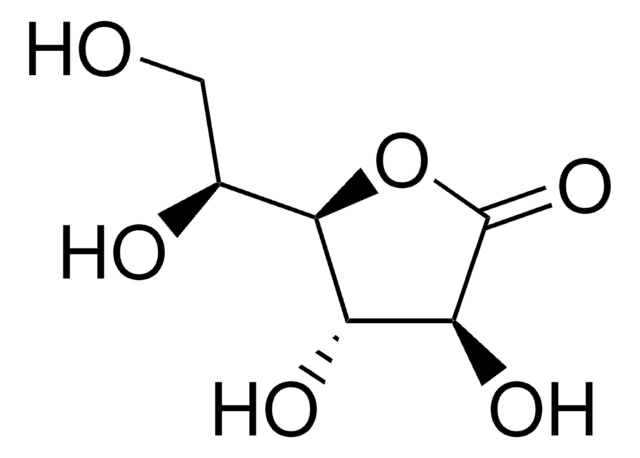
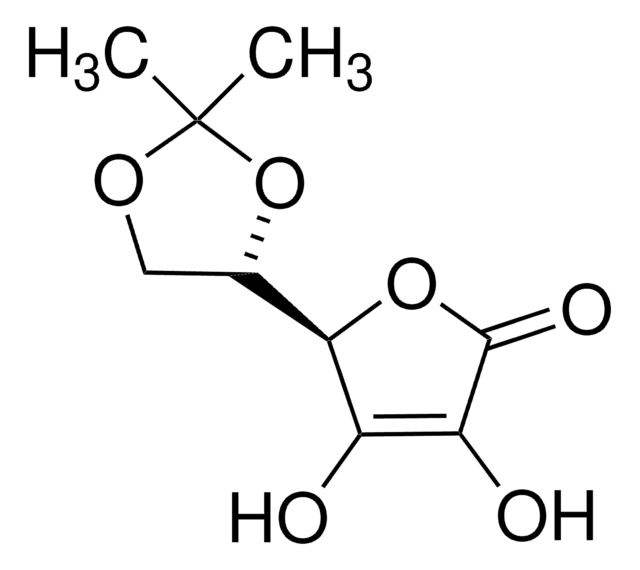
D-(+)-Ribonic γ-lactone
97%
Sinónimos:
D(+)-Ribono-1,4-lactone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H8O5
Número de CAS:
Peso molecular:
148.11
Beilstein/REAXYS Number:
82057
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
crystals
optical activity
[α]24/D +18°, c = 1 in H2O
mp
85-87 °C (lit.)
SMILES string
OC[C@H]1OC(=O)[C@H](O)[C@@H]1O
InChI
1S/C5H8O5/c6-1-2-3(7)4(8)5(9)10-2/h2-4,6-8H,1H2/t2-,3-,4-/m1/s1
InChI key
CUOKHACJLGPRHD-BXXZVTAOSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Important building block for chiral acyclics, cyclopentenones, and oxabicyclic systems. Also employed in studies on nonlinear optical materials.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Chemistry of Materials, 5, 802-802 (1993)
Aldrichimica Acta, 22, 49-49 (1989)
Tae Woo Kim et al.
Organic letters, 6(22), 3949-3952 (2004-10-22)
[structure: see text] We describe a series of nonpolar nucleoside analogues having similar shapes and gradually increasing size. The structure of the nucleobase thymine was mimicked with toluene derivatives, replacing O2/O4 with hydrogen, fluorine, chlorine, bromine, and iodine. Glycosidic bonds
Cheng-Hung Jen et al.
Nucleosides, nucleotides & nucleic acids, 29(7), 523-534 (2010-07-01)
A thorough study for the synthesis of 1-deazauridine is described. 3-Bromo-2,6-dimethoxy-5-(beta-D-ribofuranosyl)pyridine, a synthetic precursor for 1-deazauridine, was prepared in seven steps from 2,6-dimethoxypyridine and d-ribose via the ribonolactone approach. Subsequent demethylation was unsuccessful but led to presumable anomerization and isomerization.
B A Horenstein et al.
Biochemistry, 32(28), 7089-7097 (1993-07-20)
A new approach to understanding transition-state structure is presented which involves the sequential application of experimental and computational methods. A family of experimentally determined kinetic isotope effects is fit simultaneously in a vibrational analysis to provide a geometric model of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico