670774
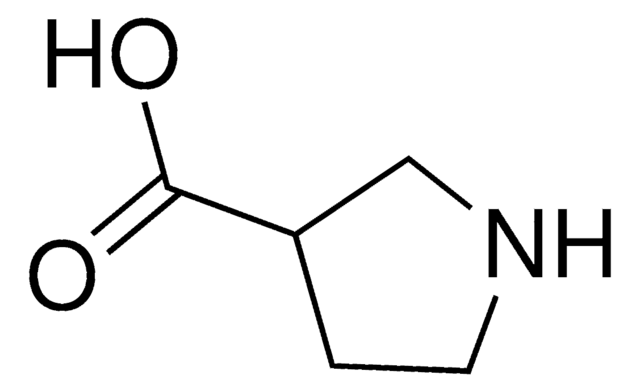
(R)-(−)-Pyrrolidine-3-carboxylic acid
≥99.0% (NT)
Sinónimos:
(R)-β-Proline
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H9NO2
Número de CAS:
Peso molecular:
115.13
Beilstein/REAXYS Number:
5496144
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Productos recomendados
assay
≥99.0% (NT)
form
solid
optical activity
[α]/D -20.5±1.5°, c = 2 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
OC(=O)[C@@H]1CCNC1
InChI
1S/C5H9NO2/c7-5(8)4-1-2-6-3-4/h4,6H,1-3H2,(H,7,8)/t4-/m1/s1
InChI key
JAEIBKXSIXOLOL-SCSAIBSYSA-N
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
David Steer et al.
Biochemistry, 41(35), 10819-10826 (2002-08-28)
The enzyme EC 3.4.24.15 (EP 24.15) is a zinc metalloendopeptidase whose precise function in vivo remains unknown but is thought to participate in the regulated metabolism of a number of specific neuropeptides. The lack of stable and selective inhibitors has
Wesley R R Harker et al.
Organic & biomolecular chemistry, 10(7), 1406-1410 (2012-01-05)
α-Alkyl β-amino esters are available in high diastereoselectivity through a silicon-free Claisen enolate [3,3]-sigmatropic rearrangement of enamide esters. Optimisation studies have probed the crucial role of the initial enolisation and the nature of the enamide N-centre. The demonstration of chirality
Haile Zhang et al.
Journal of the American Chemical Society, 130(3), 875-886 (2008-01-01)
The development of enantioselective anti-selective Mannich-type reactions of aldehydes and ketones with imines catalyzed by 3-pyrrolidinecarboxylic acid and related pyrrolidine derivatives is reported in detail. Both (3R,5R)-5-methyl-3-pyrrolidinecarboxylic acid and (R)-3-pyrrolidinecarboxylic acid efficiently catalyzed the reactions of aldehydes with alpha-imino esters
Cody Timmons et al.
The Journal of organic chemistry, 70(19), 7634-7639 (2005-09-10)
[reaction: see text] A new halo-Mannich-type reaction is reported using cyclopropyl carbonyl-derived enolates and sulfonyl-protected imines. Chiral oxazolidinones auxiliaries were found to be effective for completely controlling the stereochemistry of the products. Variations in the oxazolidinone, protecting group, and imine
Souvik Banerjee et al.
The Journal of organic chemistry, 77(23), 10925-10930 (2012-11-07)
A straightforward stereoselective and enantiodivergent cyclization strategy for the construction of γ-lactams is described. The cyclization strategy makes use of chiral malonic esters prepared from enantiomerically enriched monoesters of disubstituted malonic acid. The cyclization occurs with the selective displacement of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico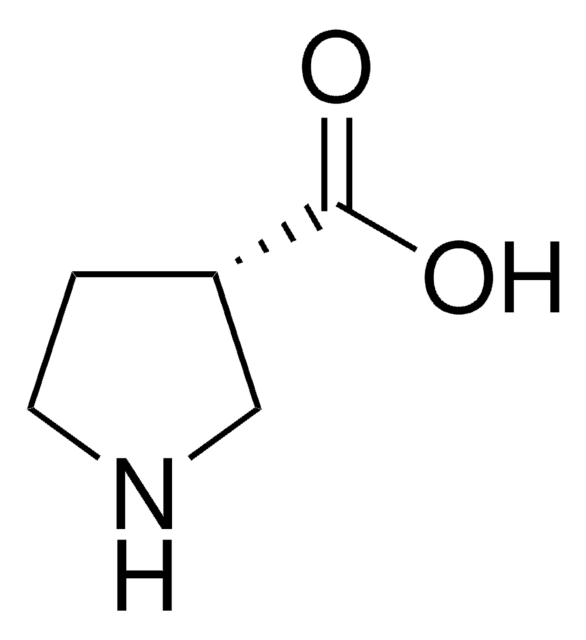

![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)