489174
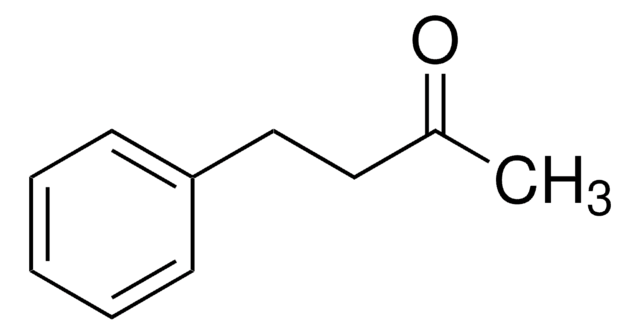
Benzyloxyacetone
90%
Sinónimos:
1-Benzyloxy-2-propanone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH2OCH2COCH3
Número de CAS:
Peso molecular:
164.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
90%
impurities
3% (benzyloxy)acetic acid
3% benzyl alcohol
refractive index
n20/D 1.512 (lit.)
bp
259 °C (lit.)
density
1.04 g/mL at 25 °C (lit.)
functional group
ether
ketone
phenyl
SMILES string
CC(=O)COCc1ccccc1
InChI
1S/C10H12O2/c1-9(11)7-12-8-10-5-3-2-4-6-10/h2-6H,7-8H2,1H3
InChI key
YHMRKVGUSQWDGZ-UHFFFAOYSA-N
General description
Benzyloxyacetone (α-Benzyloxyacetone) is an α-substituted acetone. It undergoes direct aldol reaction with 4-nitrobenzaldehyde in the presence of (S)-BINAM-L-prolinamide/benzoic acid to form predominantly the syn-diasteroisomer.
Application
Benzyloxyacetone (1-Benzyloxy-2-propanone) may be used in the synthesis of:
- 7-benzyloxy-6-methyl-5-hepten-1-yne
- (Z)-2-methylhept-2-en-6-yn-1-o1
- (S)-(+)-1,2-propanediol, 1-benzyl ether
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Synthesis and biological evaluation of L-and D-configurations of 2',3'-dideoxy-4'-C-methyl-3'-oxacytidine analogues.
Liu MC, et al.
Bioorganic & Medicinal Chemistry Letters, 11(17), 2301-2304 (2001)
A stereoselective formation of (Z)-2-methyl-2-alkenol by the wittig reaction: its application to a synthesis of nerylacetone and (Z,Z)-farnesylacetone.
Sato K, et al.
Chemistry Letters (Jpn), 10(12), 1711-1714 (1981)
Cis selective wittig olefination of a-alkoxy ketones and its application to the stereoselective synthesis of plaunotol.
Inoue S, et al.
Bulletin of the Chemical Society of Japan, 63(6), 1629-1635 (1990)
Highly selective direct aldol reaction organocatalyzed by (S)-BINAM-L-prolinamide and benzoic acid using a-chalcogen-substituted ketones as donors.
Guillena G, et al.
ARKIVOC (Gainesville, FL, United States), 4, 260-269 (2007)
Stereochemical control of bakers' yeast mediated reduction of a protected 2-hydroxy ketone.
Manzocchi A, et al.
The Journal of Organic Chemistry, 53(18), 4405-4407 (1988)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico