479896
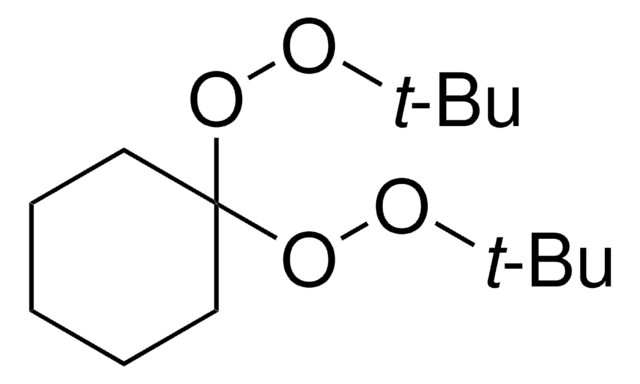
Luperox® 231, 1,1-Bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane
92%
Sinónimos:
1,1-Bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane
About This Item
Productos recomendados
Quality Level
assay
92%
refractive index
n20/D 1.441 (lit.)
mp
≥50 °C (SADT) (lit.)
density
0.904 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC1CC(C)(C)CC(C1)(OOC(C)(C)C)OOC(C)(C)C
InChI
1S/C17H34O4/c1-13-10-16(8,9)12-17(11-13,20-18-14(2,3)4)21-19-15(5,6)7/h13H,10-12H2,1-9H3
InChI key
NALFRYPTRXKZPN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 4 - Org. Perox. B
Storage Class
4.1A - Other explosive hazardous materials
wgk_germany
WGK 2
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![Bicyclo[2.2.1]hepta-2,5-diene 98%](/deepweb/assets/sigmaaldrich/product/structures/304/819/dfa7c176-c370-4fb5-acf1-28d751241a50/640/dfa7c176-c370-4fb5-acf1-28d751241a50.png)