457698
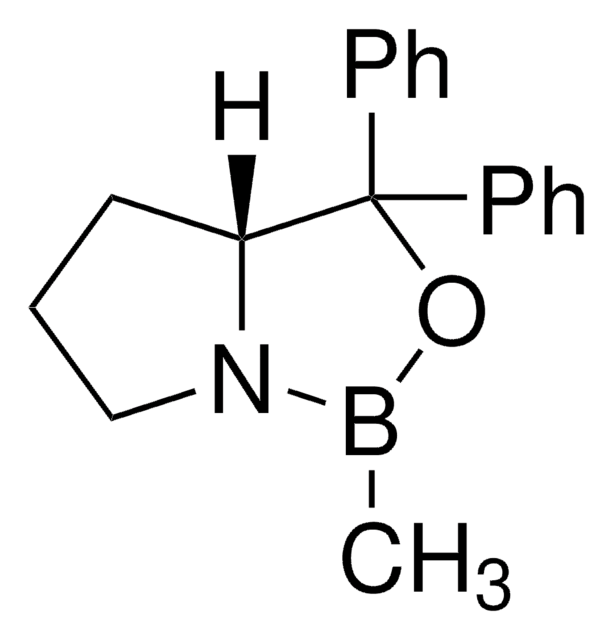
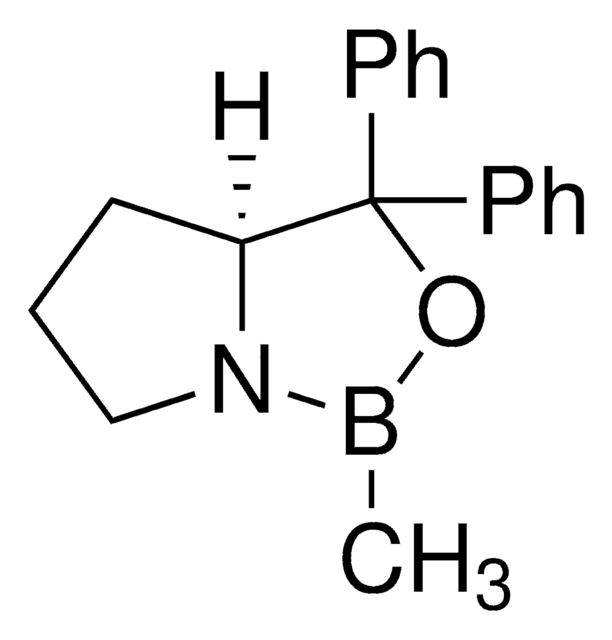
(R)-(+)-2-Methyl-CBS-oxazaborolidine solution
1 M in toluene
Sinónimos:
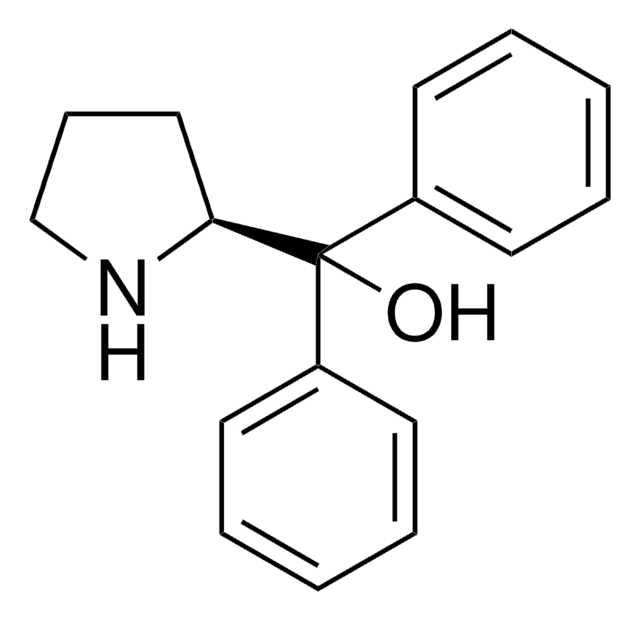
α,α-Diphenyl-D-prolinolmethylboronic acid cyclamide ester, (R)-1-Methyl,3,3-diphenyl-tetrahydro-pyrrolo(1,2-c)(1,3,2)oxazaborole, (R)-Tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborole
About This Item
Productos recomendados
Quality Level
concentration
1 M in toluene
bp
111 °C
density
0.95 g/mL at 25 °C
functional group
phenyl
storage temp.
room temp
SMILES string
[H][C@]12CCCN1B(C)OC2(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m1/s1
InChI key
VMKAFJQFKBASMU-QGZVFWFLSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
It may also be used in the preparation of:
- (-)-diospongin B
- (1R)-2-azido-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanol
- (S)-α-deuteriobenzyl alcohol
- (3S,4R,5S)-1-(trimethylsilyl)-4,5-epoxyhex-1-yn-3-ol
Used in a desymmetrizing reduction leading to (S)-4-hydroxycyclohexenone.
Physical form
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
target_organs
Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
39.2 °F - closed cup
flash_point_c
4 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We are pleased to offer o-tolyl-CBS-oxazaborolidine as a 0.5 M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide, these chiral oxazaborolidines generate chiral Lewis acids, which have demonstrated great utility in the enantioselective Diels–Alder reaction.
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
Contenido relacionado
Our company is pleased to offer both enantiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1 M solution in toluene.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico