429880
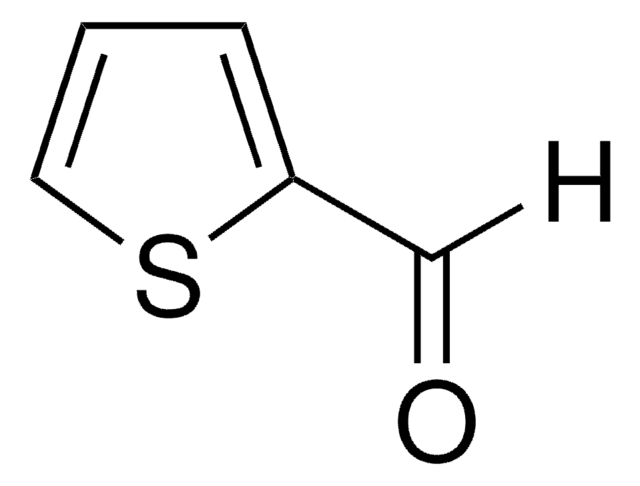
2,5-Thiophenedicarboxaldehyde
99%
Sinónimos:
2,5-Diformylthiophene, 2,5-Thienodicarboxaldehyde, 2,5-Thiophenedial, Thiophene-2,5-dialdehyde
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H4O2S
Número de CAS:
Peso molecular:
140.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
mp
115-117 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(s1)C([H])=O
InChI
1S/C6H4O2S/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChI key
OTMRXENQDSQACG-UHFFFAOYSA-N
General description
2,5-Thiophenedicarboxaldehyde can be prepared from 2,5-bis(chloromethyl)thiophene by the application of Kröhnke′s method.
Application
2,5-Thiophenedicarboxaldehyde may be employed in the following studies:
- Asymmetric synthesis of bis-homoallylic alcohols.
- Synthesis of new symmetrical arylene bisimide derivatives.
- As dialdehyde monomer in the synthesis of silicon-containing poly(p-phenylenevinylene)-related copolymers having uniform p-conjugated segment regulated by organosilicon units.
- Synthesis of 2,5-bis[2-(5-N-isopropylamidino)benzimidazoyl]thiophene hydrochloride.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Xiaoming Li et al.
Frontiers in oncology, 8, 354-354 (2018-10-16)
RNA interference (RNAi) is a biological process through which gene expression can be inhibited by RNA molecules with high selectivity and specificity, providing a promising tool for tumor treatment. Two types of molecules are often applied to inactivate target gene
M Del Poeta et al.
Antimicrobial agents and chemotherapy, 42(10), 2495-2502 (1998-10-03)
Twenty analogues of pentamidine, 7 primary metabolites of pentamidine, and 30 dicationic substituted bis-benzimidazoles were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. A majority of the compounds had MICs at which 80% of the
The Preparation of 2, 5-Thiophenedicarboxaldehyde.
Sone T.
Bulletin of the Chemical Society of Japan, 37(8), 1197-1200 (1964)
Marzena Grucela-Zajac et al.
The journal of physical chemistry. C, Nanomaterials and interfaces, 118(24), 13070-13086 (2014-06-27)
New symmetrical arylene bisimide derivatives formed by using electron-donating-electron-accepting systems were synthesized. They consist of a phthalic diimide or naphthalenediimide core and imine linkages and are end-capped with thiophene, bithiophene, and (ethylenedioxy)thiophene units. Moreover, polymers were obtained from a new
Guang-Ming Chen et al.
The Journal of organic chemistry, 64(3), 721-725 (2001-10-25)
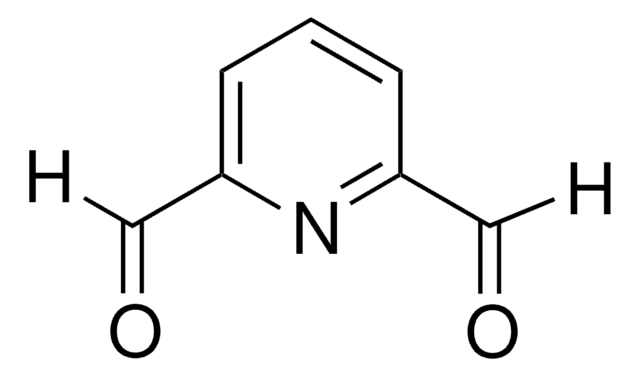
Asymmetric reduction of 2,6-diacylpyridines with B-chlorodiisopinocampheylborane provides the corresponding C(2)-symmetric diols in very high de and ee. Asymmetric allylboration of 2,6-pyridinedicarboxaldehyde and 2,5-thiophenedicarboxaldehyde provides the corresponding bis-homoallylic alcohols in very high de and ee. These optically pure diols were converted
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)