371475
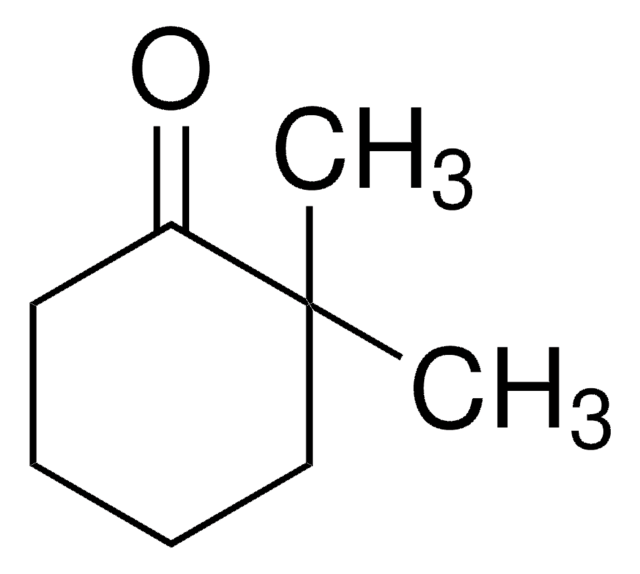
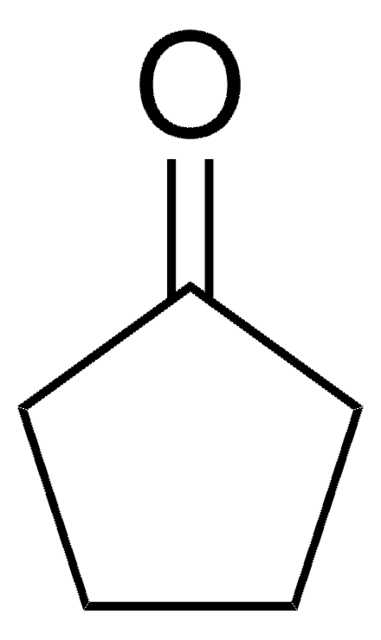
2,2-Dimethylcyclopentanone
96%
About This Item
Productos recomendados
Quality Level
assay
96%
refractive index
n20/D 1.433 (lit.)
bp
143-145 °C (lit.)
density
0.894 g/mL at 25 °C (lit.)
SMILES string
CC1(C)CCCC1=O
InChI
1S/C7H12O/c1-7(2)5-3-4-6(7)8/h3-5H2,1-2H3
InChI key
FTGZMZBYOHMEPS-UHFFFAOYSA-N
Categorías relacionadas
General description
Application
- 2,6,6-trimethyl-2-azaspiro[4.4]nonane-1,3-dione, a spirosuccinimide moiety of asperparaline A
- novel spiropentanopyrrolizidine oxime alkaloids, namely 2′,3′,5′,6′,7′,7a′-hexahydro-2,2-dimethylspirocyclopentane-1
- δ,δ-dimethyl-δ-valerolactone, via Baeyer-Villiger oxidation
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico