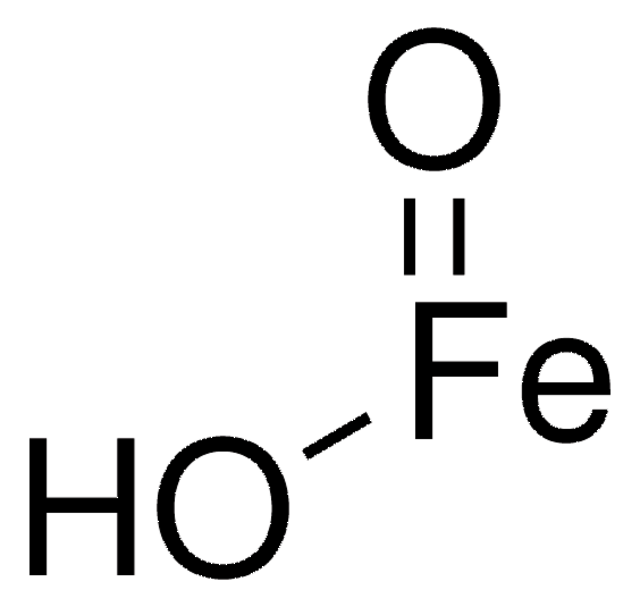
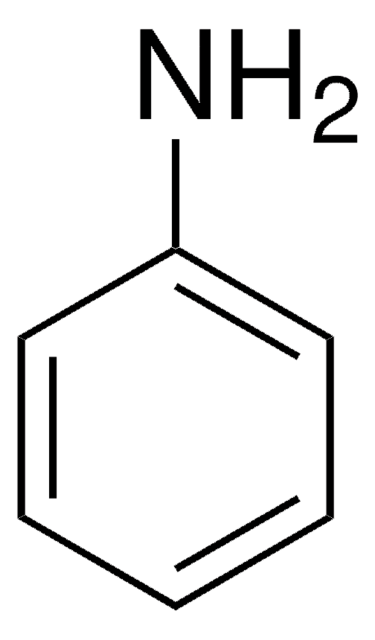
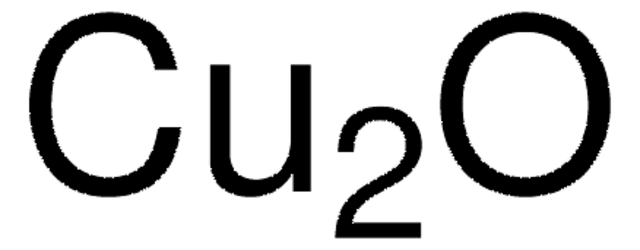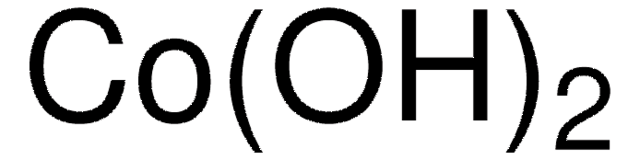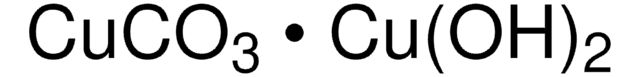289787
Copper(II) hydroxide
technical grade
Sinónimos:
Cupric hydroxide
About This Item
Productos recomendados
grade
technical grade
form
powder
contains
stabilizer
concentration
≥57.0% Cu (EDTA titration)
solubility
H2O: insoluble(lit.)
aqueous acid: slightly soluble(lit.)
application(s)
battery manufacturing
SMILES string
O[Cu]O
InChI
1S/Cu.2H2O/h;2*1H2/q+2;;/p-2
InChI key
JJLJMEJHUUYSSY-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Storage Class
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico