276286
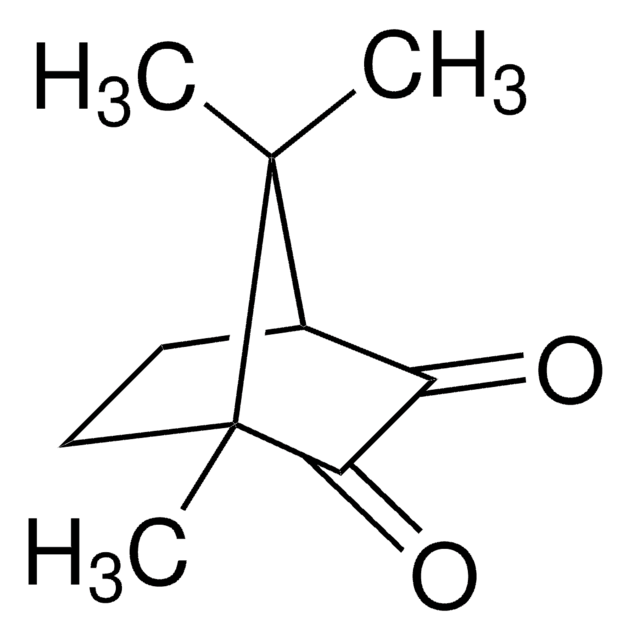
(1R)-(−)-Camphorquinone
99%
Sinónimos:
(1R)-(−)-2,3-Bornanedione, 2,3-Bornanedione
About This Item
Productos recomendados
Quality Level
assay
99%
optical activity
[α]20/D −101°, c = 2 in toluene
mp
200-203 °C (lit.)
functional group
ketone
SMILES string
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2=O
InChI
1S/C10H14O2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11/h6H,4-5H2,1-3H3/t6-,10+/m1/s1
InChI key
VNQXSTWCDUXYEZ-LDWIPMOCSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- α-Hydroxycamphors by selective reduction of keto groups using various vegetables.
- Camphor-1,2-diamine platinum(II) complexes for DNA interaction studies.
- Camphoric anhydride by unsensitized photo-oxidation in the presence of oxygen and polar solvents.
- Camphorquinone-based chiral homoallylic amine, which is reacted with aldehydes to produce homoallylic primary amines via imine formation followed by 2-azonia-Cope rearrangement.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico