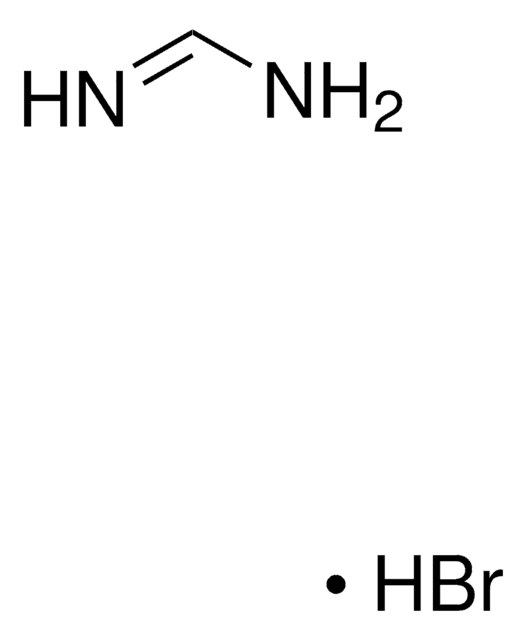
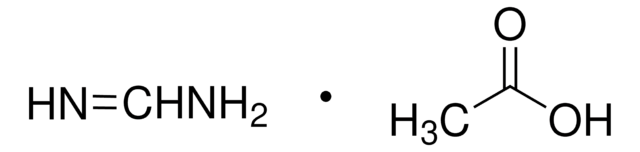268607
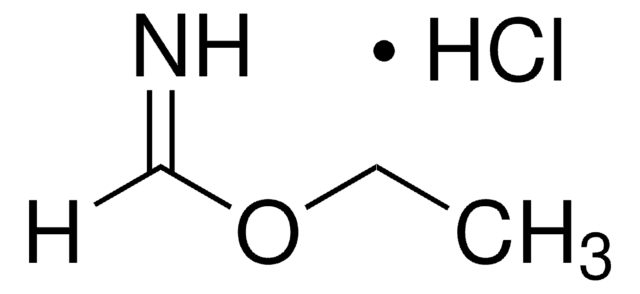
Formamidine hydrochloride
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HN=CHNH2 · HCl
Número de CAS:
Peso molecular:
80.52
Beilstein/REAXYS Number:
3906935
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
mp
84-87 °C (lit.)
functional group
amine
SMILES string
Cl[H].[H]C(N)=N
InChI
1S/CH4N2.ClH/c2-1-3;/h1H,(H3,2,3);1H
InChI key
NMVVJCLUYUWBSZ-UHFFFAOYSA-N
Application
Formamidine hydrochloride was used in the synthesis of imidazoleglycerol phosphate (IGP). It was also used in the synthesis of 5-methyl-4,6-dihydroxypyrimidine.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
The biosynthesis of histidine; D-erythro-imidazoleglycerol phosphate dehydrase.
B N AMES
The Journal of biological chemistry, 228(1), 131-143 (1957-09-01)
Reactions of oxidizing radicals with 4, 6-dihydroxypyrimidines as model compounds for uracil, thymine, and cytosine.
The Journal of Physical Chemistry, 91(2), 426-433 (1987)
Federico Bertasi et al.
Physical chemistry chemical physics : PCCP, 19(38), 26230-26239 (2017-09-22)
This work describes the preparation of the new lipophilic ionic liquid tetraoctyl-formamidinium bis(trifluoromethanesulfonyl) imide (TOFATFSI), which is miscible with lower alkanes. In particular, this work focuses on the electric behaviour of TOFATFSI in the particularly challenging highly apolar environment of
José M Casas et al.
Inorganic chemistry, 44(25), 9444-9452 (2005-12-06)
The preparation of the [NBu4][Pt(C6F5)3L] complexes (L=triazene, formamidine, 2-aminopyridine,) have been carried out. These ligands contain a hydrogen atom, with more or less acidic character, in a position suitable for establishing an intramolecular hydrogen bonding interaction with the metal center.
Carsten Präsang et al.
Journal of the American Chemical Society, 127(29), 10182-10183 (2005-07-21)
Carbene analogues of borazines are highly thermally stable. Keeping quasi-identical steric demands, the electronic properties of the carbene can be precisely tuned by varying the nature of the substituents at the boron centers.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico