229601
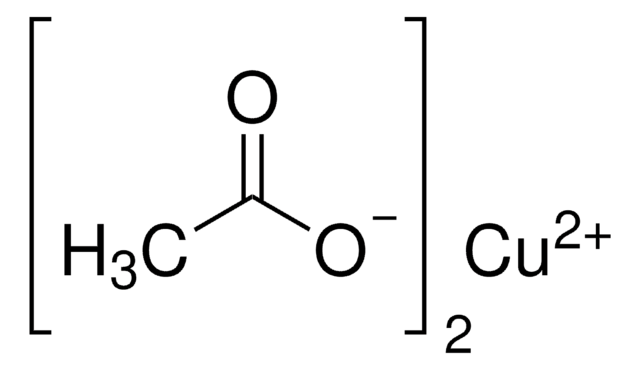
Copper(II) acetate monohydrate
99.99% trace metals basis
Sinónimos:
Cupric acetate monohydrate
About This Item
Productos recomendados
vapor density
6.8 (vs air)
Quality Level
assay
99.99% trace metals basis
form
powder or crystals
reaction suitability
core: copper
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
SMILES string
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
InChI key
NWFNSTOSIVLCJA-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- To synthesize CuSbS2 nanoplates and a CuSbS2-Cu3SbS4 nanocomposite via hot injection method. CuSbS2 can be used as an absorber material in solar cells due to its favorable optical properties and direct band gap. The CuSbS2-Cu3SbS4 nanocomposite exhibits promising super capacitive properties, making it suitable for energy storage applications.
- To synthesize copper oxide nanoparticles (CuO NPs) using a green synthesis method involving psidium guajava leaf extract as both a reducing and capping agent. The CuO NPs exhibit excellent photocatalytic activity for degrading industrial dyes, such as Nile Blue (NB) and Reactive Yellow 160 (RY160). These nanoparticles can be used for purifying water resources contaminated with industrial dyes.
- As a copper precursor in synthesizing CuO semiconducting thin films via jet nebulizer spray pyrolysis technique, for P–N diode application. These CuO films find applications in supercapacitors, sensors, solar cells, photocatalysis and electrochromic devices.
Features and Benefits
- It is soluble in water makes a perfect precursor for the synthesis of new materials by sol-gel method
- With high purity of 99.99% (<150 ppm) and low heavy metals, it is ideal for Ir-catalyzed intramolecular C-H amination and the synthesis of carbinol.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
does not flash
flash_point_c
does not flash
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico