224286
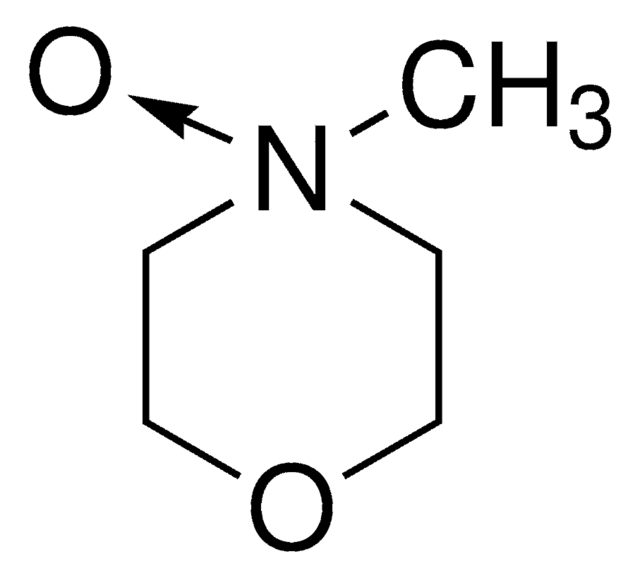
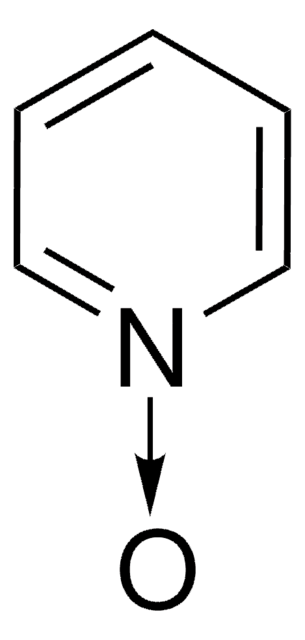
4-Methylmorpholine N-oxide
97%
Sinónimos:
NMO
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H11NO2
Número de CAS:
Peso molecular:
117.15
Beilstein/REAXYS Number:
507437
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
reaction suitability
reagent type: oxidant
mp
180-184 °C (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
C[N+]1([O-])CCOCC1
InChI
1S/C5H11NO2/c1-6(7)2-4-8-5-3-6/h2-5H2,1H3
InChI key
LFTLOKWAGJYHHR-UHFFFAOYSA-N
General description
4-Methylmorpholine N-oxide is an organic compound used as a co-oxidant along with OsO4 and ruthenates in organic synthesis. In recent studies, it has been used as a catalyst in silylcyanation of aldehydes and ketones. Lyocell, a regenerated cellulose fiber, can be prepared using 4-methylmorpholine N-oxide in an eco-friendly manner.
Application
Non-metallic catalyst for the cyanosilylation of ketones. Co-oxidant for Sharpless asymmetric dihydroxylation in ionic liquids.
signalword
Danger
hcodes
Hazard Classifications
Flam. Sol. 1 - Repr. 2
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synlett, 6, 1077-1079 (2004)
Marzieh Shafiei et al.
Bioresource technology, 102(17), 7879-7886 (2011-06-21)
Given that N-methylmorpholine-N-oxide (NMMO) is a promising alternative for the pretreatment of lignocelluloses, a novel process for ethanol and biogas production from wood was developed. The solvent, NMMO, is concentrated by multistage evaporation, and the wood is pretreated with the
Nicolas Dupuy et al.
The Journal of chemical physics, 142(21), 214109-214109 (2015-06-08)
We study the ionization energy, electron affinity, and the π → π(∗) ((1)La) excitation energy of the anthracene molecule, by means of variational quantum Monte Carlo (QMC) methods based on a Jastrow correlated antisymmetrized geminal power (JAGP) wave function, developed
Luís C Branco et al.
The Journal of organic chemistry, 69(13), 4381-4389 (2004-06-19)
The use of room-temperature ionic liquids (RTILs) in the Sharpless catalytic asymmetric dihydroxylation (AD) as a cosolvent or replacement of the tert-butanol was studied in detail by screening 11 different RTILs. The AD reaction is faster in 1-n-butyl-3-methylimidazolium hexafluorophosphate [C(4)mim][PF(6)]
N?Methylmorpholine N?Oxide
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2008)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico