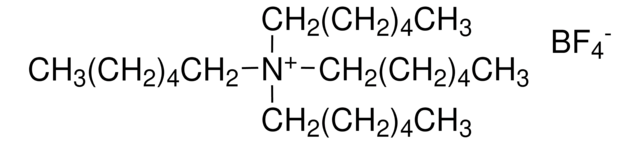217964
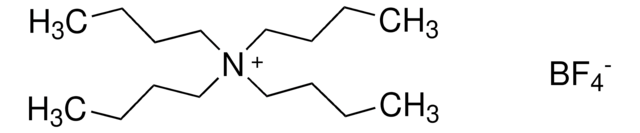
Tetrabutylammonium tetrafluoroborate
99%
Sinónimos:
Ammonium tetra-n-butyl tetrafluoroborate
About This Item
Productos recomendados
Quality Level
assay
99%
form
powder
mp
155-161 °C (lit.)
solubility
methanol: soluble 10%, clear, colorless
SMILES string
F[B-](F)(F)F.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.BF4/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;2-1(3,4)5/h5-16H2,1-4H3;/q+1;-1
InChI key
NNZZSJSQYOFZAM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Tetrabutylammonium tetrafluoroborate (TBATFB) is a phase transfer catalyst. It can be synthesized by the reaction between 30% aqueous solution of tetrafluoroboric acid and 40% aqueous solution of tetrabutylamonium hydroxide. Tetrabutylammonium tetrafluoroborate acts as an electrolyte and inhibits the self-assembly of alkylthiosulfate on gold.
Application
- As supporting electrolyte in the voltammetric determination of Δ(9)-tetrahydrocannabinol (Δ(9)-THC).
- Synthesis of biologically relevant macrolactones, Sansalvamide A.
- As supporting electrolyte in the determination of the oxidation and reduction potentials of 5,10,15,20-tetra[3-(3-trifluoromethyl)phenoxy]porphyrin by cyclic voltammetry.
- Preparation of 1:1 adduct with 1,10-phenanthroline.
- Used to prepare other tetrabutylammonium salts in aqueous solutions.
- As electrolyte additive in the synthesis of conducting poly(thiophenes).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
No data available
flash_point_c
No data available
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico