196282
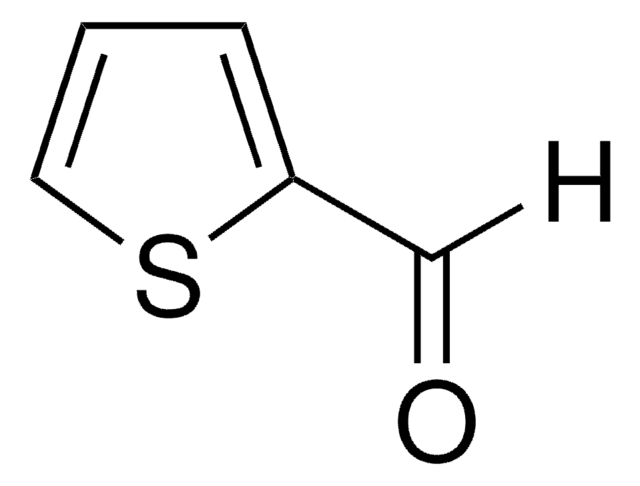
3-Thiophenecarboxaldehyde
98%
Sinónimos:
3-Formylthiophene, 3-Thienaldehyde, 3-Thienylcarboxaldehyde, 3-Thiophenealdehyde, 3-Thiophenecarbaldehyde, Thiofuran-3-carboxaldehyde, Thiophen-3-aldehyde
About This Item
Productos recomendados
vapor pressure
0.31 mmHg ( 20 °C)
assay
98%
form
liquid
autoignition temp.
>392 °F
refractive index
n20/D 1.583 (lit.)
bp
194-196 °C (lit.)
86-87 °C/20 mmHg (lit.)
density
1.28 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccsc1
InChI
1S/C5H4OS/c6-3-5-1-2-7-4-5/h1-4H
InChI key
RBIGKSZIQCTIJF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- series of 4-substituted 2-thiophenesulfonamides
- acetal and ketal derivatives of 4′-demethylepipodophyllotoxin-β-D-glucoside and epipodophyllotoxin-β-D-glucoside
- 1,2-di-3-thienyl-2-hydroxyethanone (3,3′-thenoin), 3-thienyl symmetric analog of benzoin
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico