192694
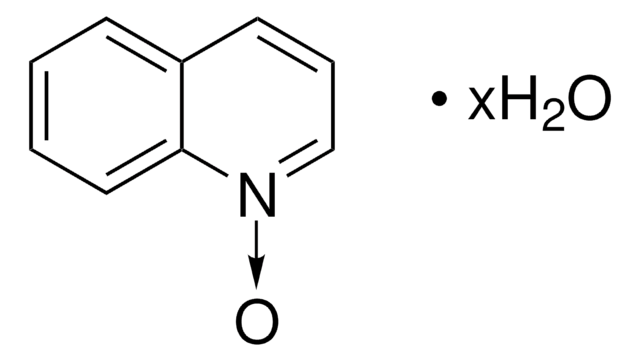
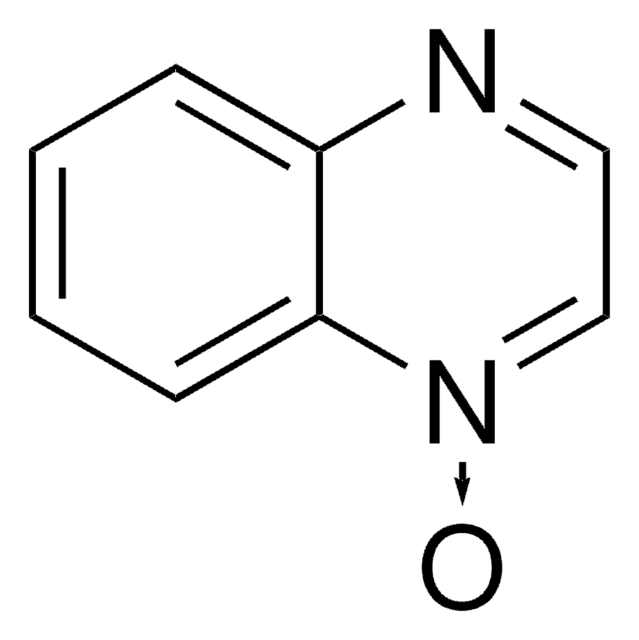
Isoquinoline N-oxide
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H7NO
Número de CAS:
Peso molecular:
145.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
solid
mp
103-105 °C (lit.)
SMILES string
[O-][n+]1ccc2ccccc2c1
InChI
1S/C9H7NO/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-7H
InChI key
RZIAABRFQASVSW-UHFFFAOYSA-N
General description
Photochemical isomerization of isoquinoline N-oxide in methanol or water has been investigated by steady-light irradiation and flash spectroscopy. It is a useful intermediate for isoquinoline derivatives. It causes the oxidation of fullerene C60 under ultrasonic irradiation in air.
Application
Isoquinoline N-oxide was used in the synthesis of N-alkoxy isoquinolinium and N-alkoxy 4-phenylpyridinium ion terminated polystyrenes.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Weon-Bae Ko et al.
Ultrasonics, 42(1-9), 611-615 (2004-03-30)
The reaction of C60 with various amine N-oxides such as 3-picoline N-oxide (Aldrich 98.0%), pyridine N-oxide hydrate (Aldrich 95.0%), quinoline N-oxide (Aldrich 97.0%), isoquinoline N-oxide (Aldrich 98.0%) under ultrasonic irradiation in air at 25-43 degrees C causes the oxidation of
N-alkoxy pyridinium ion terminated polystyrenes: A facile route to photoinduced block copolymerization.
Durmaz YY, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 45(3), 423-428 (2007)
The primary photochemical process of isoquinoline N-oxide in hydroxylic solvents.
Ono I and Hata N.
Bulletin of the Chemical Society of Japan, 46, 3658-3662 (1973)
Iodine-mediated electrophilic cyclization of 2-alkynylbenzaldoximes leading to the formation of iodoisoquinoline N-oxides.
Huo Z, et al.
Tetrahedron Letters, 49(38), 5531-5533 (2008)
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico