185361
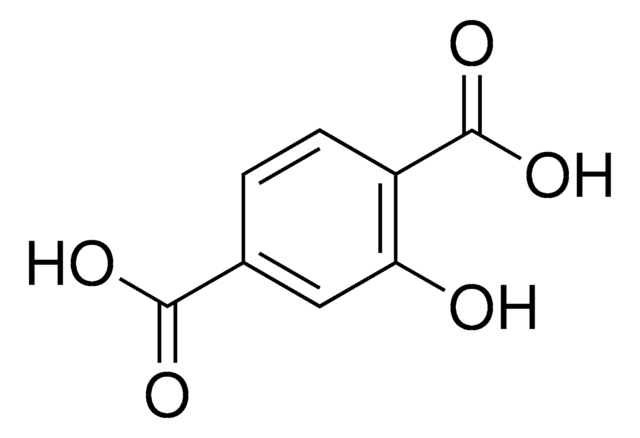
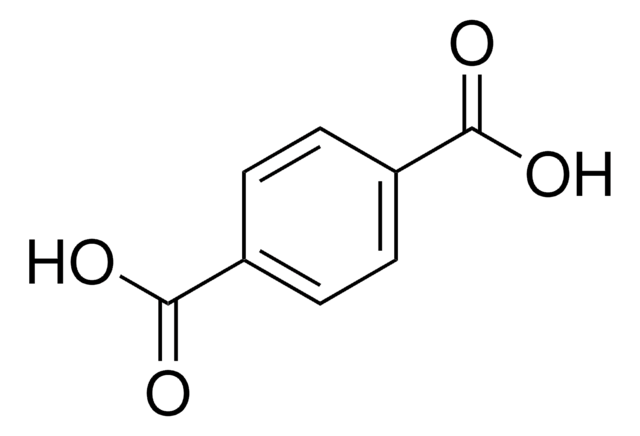
Terephthalic acid
98%
Sinónimos:
Benzene-1,4-dicarboxylic acid
About This Item
Productos recomendados
vapor pressure
<0.01 mmHg ( 20 °C)
assay
98%
form
powder
autoignition temp.
925 °F
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>300 °C (lit.)
solubility
water: ~0.017 g/L at 25 °C
density
1.58 g/cm3 at 25 °C
greener alternative category
SMILES string
OC(=O)c1ccc(cc1)C(O)=O
InChI
1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
InChI key
KKEYFWRCBNTPAC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
- As a monomer in the synthesis of poly(butylene terephthalate) (PBT), a type of polyester, that is used in various fields including Automotive components, textile Industry, packaging materials, electrical and electronic components.
- As an organic ligand in the synthesis of the cobalt(II) metal–organic framework (MOFs), which finds applications in electrochemical energy storage, catalysis, optoelectronics, and water treatment.
- Terephthalic acid (TPA) can be synthesized from bio-based materials for a variety of applications, which include the production of polyester fiber, non-fiber field, PET bottles, synthetic perfumes and medicines.
- Terephthalic acid is used as a linker molecule in the preparation of metal-organic frameworks (MOFs).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico