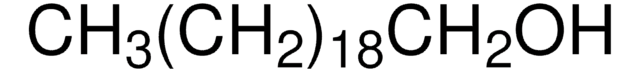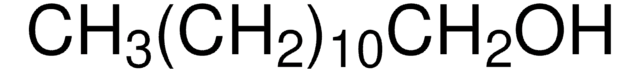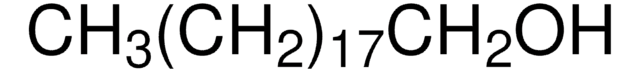169102
1-Docosanol
98%
Sinónimos:
Behenyl alcohol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3(CH2)21OH
Número de CAS:
Peso molecular:
326.60
Beilstein/REAXYS Number:
1770470
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
bp
180 °C/0.22 mmHg (lit.)
mp
65-72 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
InChI key
NOPFSRXAKWQILS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Application
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
410.0 °F
flash_point_c
210 °C
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Ai-Mei Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 32(10), 1534-1537 (2010-02-02)
To study the chemical constituents from Clematis brevicaudata. The compounds were isolated by column chromatography and their structures were elucidated through spectroscopic analysis (NMR). Eight compounds were isolated and identified as: palmitic acid (1), 1-docosanol (2), pentacosanoic acid-2', 3'-dihydroxypropyl ester
Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores.
Cho B-K, et al.
Macromolecules, 37(11), 4227-4234 (2004)
Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex.
D H Katz et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825-10829 (1991-12-01)
This article reports that 1-docosanol, a 22-carbon-long saturated alcohol, exerts a substantial inhibitory effect on replication of certain viruses (e.g., herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. To study the basis for its viral
John F Marcelletti
Antiviral research, 56(2), 153-166 (2002-10-09)
Interactions between docosanol (n-docosanol, behenyl alcohol) and nucleoside or pyrophosphate analogs were investigated in vitro. The anti-HSV activity of acyclovir (ACV) was synergistically enhanced by treatment of cells with docosanol as judged by inhibition of progeny virus production and plaque
Clara L Shaw et al.
Journal of chemical ecology, 37(4), 329-339 (2011-03-23)
The uropygial secretions of some bird species contain volatile and semivolatile compounds that are hypothesized to serve as chemical signals. The abundance of secretion components varies with age and season, although these effects have not been investigated in many species.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico