157872
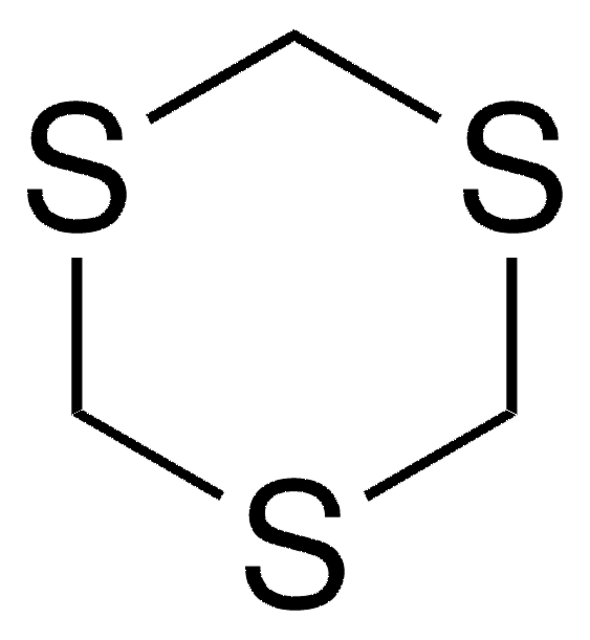
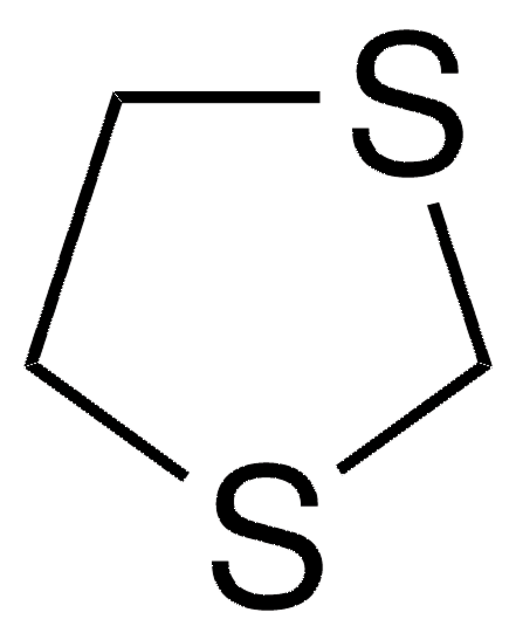
1,3-Dithiane
97%
Sinónimos:
m-Dithiane (7CI), m-Dithiane (8CI)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H8S2
Número de CAS:
Peso molecular:
120.24
Beilstein/REAXYS Number:
102534
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
solid
mp
52-54 °C (lit.)
functional group
thioether
SMILES string
C1CSCSC1
InChI
1S/C4H8S2/c1-2-5-4-6-3-1/h1-4H2
InChI key
WQADWIOXOXRPLN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
1,3-Dithiane, a protected formaldehyde anion equivalent, serves as useful labeled synthon.
Application
1,3-Dithiane was used as reagent for deoxygenation of sulfoxides to their corresponding sulfides.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
194.0 °F - closed cup
flash_point_c
90 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
A I Noskov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(1), 6-10 (2010-07-17)
The IR spectra of 1,3-dithiane-1-oxide (I) and 1,3-dithia-1-oxocyclohept-5-ene (II) were recorded in solution, solid and liquid phase over 4000-400 cm(-1) spectral range. It was found that both (I) and (II) in liquid phase and solutions exist in two conformations: (I)
Yuncong Chen et al.
Chemical communications (Cambridge, England), 48(42), 5094-5096 (2012-04-20)
A novel sensitive and specific Hg(2+) chemodosimeter, derived from 1',3'-dithiane-substituted 2,1,3-benzoxadiazole, displays "turn-on" fluorescent and colorimetric responses via an Hg(2+)-triggered aldehyde recovery reaction. Its potential to monitor Hg(2+) in living organisms has been demonstrated using zebrafish larvae.
Nasser Iranpoor et al.
The Journal of organic chemistry, 67(9), 2826-2830 (2002-04-27)
A new, mild, and novel method is described for the efficient deoxygenation of sulfoxides to their corresponding sulfides with 1,3-dithiane at room temperature in the presence of catalytic amounts of N-bromosuccinimide (NBS), 2,4,4,6-tetrabromo-2,5-cyclohexadienone (TABCO), or Br(2) as the source of
Al-Monsur Jiaul Haque et al.
Chemical communications (Cambridge, England), (32)(32), 4865-4867 (2009-08-05)
We report the use of 1,3-dithiane combined with aryldiazonium cation for the immobilization of biomolecules based on electrochemical addressing.
Valerie J Peterson et al.
The Biochemical journal, 362(Pt 1), 173-181 (2002-02-07)
Apo and holo forms of retinoic acid receptors, and other nuclear receptors, display differential sensitivity to proteolytic digestion that likely reflects the distinct conformational states of the free and liganded forms of the receptor. We have developed a method for
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico