149136
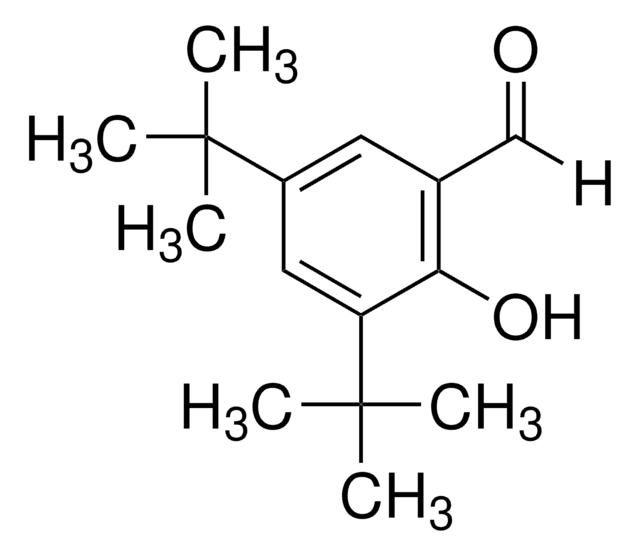
3,5-Di-tert-butylsalicylic acid
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
[(CH3)3C]2C6H2-2-(OH)CO2H
Número de CAS:
Peso molecular:
250.33
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
mp
157-162 °C (lit.)
functional group
carboxylic acid
SMILES string
CC(C)(C)c1cc(C(O)=O)c(O)c(c1)C(C)(C)C
InChI
1S/C15H22O3/c1-14(2,3)9-7-10(13(17)18)12(16)11(8-9)15(4,5)6/h7-8,16H,1-6H3,(H,17,18)
InChI key
ZWQBZEFLFSFEOS-UHFFFAOYSA-N
Categorías relacionadas
Application
3,5-Di-tert-butylsalicylic acid was used to study long wavelength fluorescence emission of 3,5-Di-tert-butylsalicylic acid in a variety of organic solvents. It was also used to catalyze the reaction between aldehydes and silyl ketene acetals.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
354.2 °F - closed cup
flash_point_c
179 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Veli T Kasumov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 107, 31-38 (2013-02-19)
A series of new polyfluorinated palladium(II) complexes (7-12) of N-polyfluorophenyl-3,5-di-tert-butylsalicylaldimines (1-6) have been synthesized. They were characterized by analytical, spectroscopic (UV/Vis, IR, (1)H NMR, and ESR), electrochemical methods and their chemical oxidation and hydrogenation catalytic activity were studied. The X-ray
Photoinduced proton transfers in 3, 5-di-tert-butylsalicylic acid.
The Journal of Physical Chemistry, 99(32), 12103-12108 (1995)
A first example of macromolecular Ti (IV) Lewis acid in the catalytic enantioselective Mukaiyama reaction.
Tetrahedron Asymmetry, 9(9), 1479-1482 (1998)
M V Chidambaram et al.
Journal of pharmaceutical sciences, 80(8), 810-811 (1991-08-01)
The initial yield of 3,5-di-t-butylsalicylic acid obtained via Kolbe-Schmitt carboxylation of the potassium salt of 2,4-di-t-butylphenol was less than 1% and was accompanied by a 65% yield of 2,2'-dihydroxy-3,3',5,5'- tetra-t-butylbiphenyl, a dimer of the 2,4-di-t-butylphenol formed by ortho coupling of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico