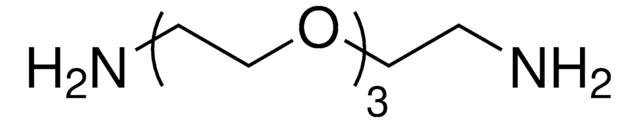
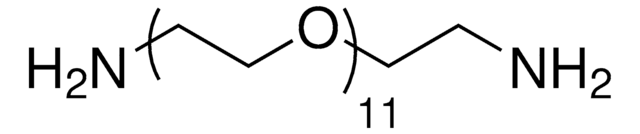
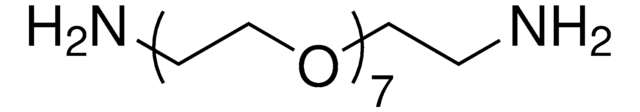14502
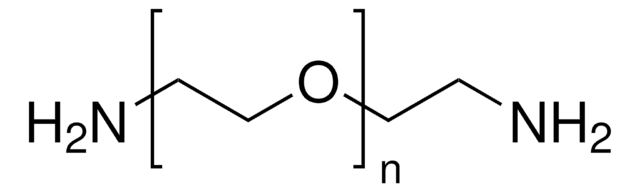
Poly(ethylene glycol) bis(amine)
Mw 3,000, carboxyl reactive, amine
Sinónimos:
Polyethylene glycol, O,O′-Bis(2-aminoethyl)polyethylene glycol, Diaminopolyethylene glycol, PEG-diamine, Polyoxyethylene bis(amine)
About This Item
Productos recomendados
product name
Poly(ethylene glycol) bis(amine), Mw 3,000
mol wt
Mw 3,000
reaction suitability
reagent type: cross-linking reagent
reactivity: carboxyl reactive
Ω-end
amine
α-end
amine
polymer architecture
shape: linear
functionality: homobifunctional
InChI
1S/C6H16N2O2/c7-1-3-9-5-6-10-4-2-8/h1-8H2
InChI key
IWBOPFCKHIJFMS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Progress in biotechnology fields such as tissue engineering and drug delivery is accompanied by an increasing demand for diverse functional biomaterials. One class of biomaterials that has been the subject of intense research interest is hydrogels, because they closely mimic the natural environment of cells, both chemically and physically and therefore can be used as support to grow cells. This article specifically discusses poly(ethylene glycol) (PEG) hydrogels, which are good for biological applications because they do not generally elicit an immune response. PEGs offer a readily available, easy to modify polymer for widespread use in hydrogel fabrication, including 2D and 3D scaffold for tissue culture. The degradable linkages also enable a variety of applications for release of therapeutic agents.
Devising biomaterial scaffolds that are capable of recapitulating critical aspects of the complex extracellular nature of living tissues in a threedimensional (3D) fashion is a challenging requirement in the field of tissue engineering and regenerative medicine.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico