116416
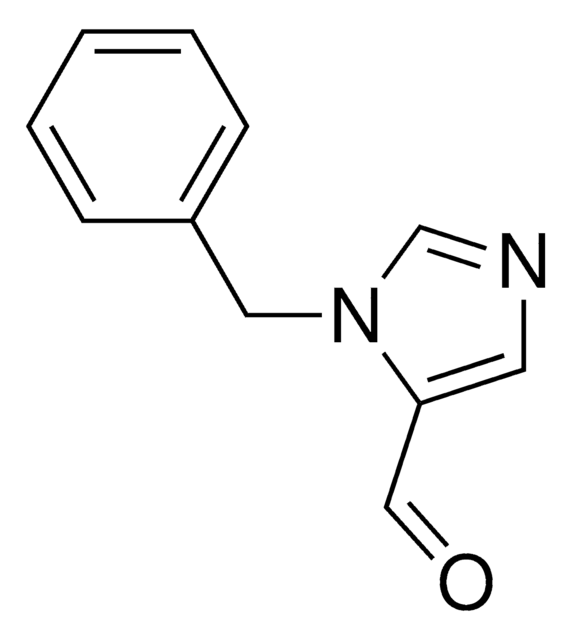
1-Benzylimidazole
99%
Sinónimos:
1-(Phenylmethyl)-1H-imidazole, 1-Benzyl-1H-imidazole, N-Benzylimidazole
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H10N2
Número de CAS:
Peso molecular:
158.20
Beilstein/REAXYS Number:
114571
EC Number:
MDL number:
UNSPSC Code:
12352005
eCl@ss:
39161001
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
bp
310 °C (lit.)
mp
68-70 °C (lit.)
functional group
phenyl
SMILES string
C(c1ccccc1)n2ccnc2
InChI
1S/C10H10N2/c1-2-4-10(5-3-1)8-12-7-6-11-9-12/h1-7,9H,8H2
InChI key
KKKDZZRICRFGSD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
1-Benzylimidazole has been used to prepare cyclodextrin-ionic liquid polymer (βCD-BIMOTs-TDI).
Biochem/physiol Actions
1-Benzylimidazole is a CYP inhibitor that inhibits the biotransformation of MeO-BDEs (methoxylated-brominated diphenyl ethers) to OH-BDEs (hydroxylated) in fishes.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Jérémie Doiron et al.
European journal of medicinal chemistry, 46(9), 4010-4024 (2011-06-28)
A series of bis- and mono-benzonitrile or phenyl analogues of letrozole 1, bearing (1,2,3 and 1,2,5)-triazole or imidazole, were synthesized and screened for their anti-aromatase activities. The unsubstituted 1,2,3-triazole 10a derivative displayed inhibitory activity comparable with that of the aromatase
José María Navas et al.
Environmental toxicology and chemistry, 22(4), 830-836 (2003-04-11)
Xenobiotics can induce cytochrome P4501A (CYP1A) by ligand binding to the aryl hydrocarbon receptor (AhR). Typical AhR ligands are polycyclic aromatic compounds with planar molecular conformation. The present work investigated the ability of the N-imidazole derivative, 1-benzylimidazole (BIM), to induce
P Rothenbach et al.
Journal of applied physiology (Bethesda, Md. : 1985), 83(2), 530-536 (1997-08-01)
This study examines the hypothesis that intestinal ischemia-reperfusion (I/R) injury contributes to renal dysfunction by altered renal eicosanoid release. Anesthetized Sprague-Dawley rats underwent 60 min of sham or superior mesenteric artery (SMA) occlusion with 60 min of reperfusion. The I/R
A Grothusen et al.
Archives of toxicology, 71(1-2), 64-71 (1996-01-01)
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic hetero-atoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic
Fredrik Jernerén et al.
Lipids, 47(7), 707-717 (2012-05-01)
(8R)-Hydroperoxy-(9Z,12Z)-octadecadienoic acid (8-HPODE) is formed by aspergilli as an intermediate in biosynthesis of oxylipins with effects on sporulation. 8-HPODE is transformed by separate diol synthases to (5S,8R)-dihydroxy- and (8R,11S)-dihydroxy-(9Z,12Z)-octadecadienoic acids (5,8- and 8,11-DiHODE). The former is formed by the cytochrome
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)