108995
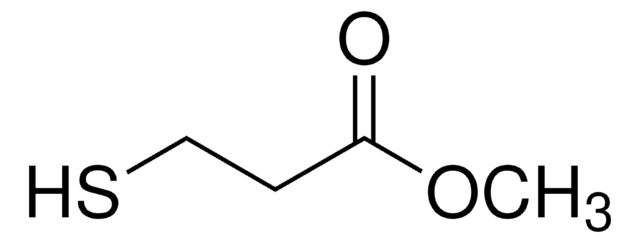
Methyl thioglycolate
95%
Sinónimos:
Methyl mercaptoacetate
About This Item
Productos recomendados
Quality Level
assay
95%
reaction suitability
reagent type: ligand
reaction type: [1,2]-Wittig Rearrangement
refractive index
n20/D 1.466 (lit.)
bp
42-43 °C/10 mmHg (lit.)
density
1.187 g/mL at 25 °C (lit.)
functional group
ester
thiol
SMILES string
COC(=O)CS
InChI
1S/C3H6O2S/c1-5-3(4)2-6/h6H,2H2,1H3
InChI key
MKIJJIMOAABWGF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- 3-carbomethoxy-4- oxotetrahydrothiopyran, 2- and 4-carbomethoxy-3-oxotetrahydrothiophene
- methyl thioglycolate and aminoethanethiol conjugated gold nanorods.
A General Strategy for Organocatalytic Activation of C–H Bonds via Photoredox Catalysis: Direct Arylation of Benzylic Ethers
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
132.8 °F - closed cup
flash_point_c
56 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico