UC179
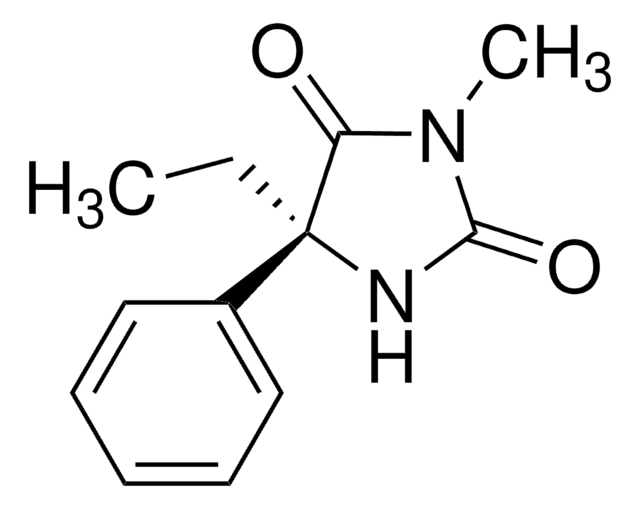
(R)-(−)-Nirvanol
Synonym(s):
(R)-(−)-5-Ethyl-5-phenyl-2,4-imidazolidinedione, (R)-(−)-5-Ethyl-5-phenylhydantoin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12N2O2
CAS Number:
Molecular Weight:
204.23
MDL number:
UNSPSC Code:
12161501
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
form
solid
Quality Level
color
off-white
solubility
DMF: soluble
storage temp.
2-8°C
SMILES string
CC[C@@]1(NC(=O)NC1=O)c2ccccc2
InChI
1S/C11H12N2O2/c1-2-11(8-6-4-3-5-7-8)9(14)12-10(15)13-11/h3-7H,2H2,1H3,(H2,12,13,14,15)/t11-/m1/s1
InChI key
UDTWZFJEMMUFLC-LLVKDONJSA-N
Biochem/physiol Actions
CYP2B6 metabolite of (S)-(+)-mephenytoin; anticonvulsant; hypnotic.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Preparation Note
Nirvanol is soluble in DMF.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W Kalow
Xenobiotica; the fate of foreign compounds in biological systems, 16(5), 379-389 (1986-05-01)
The antiepileptic drug mephenytoin is a racemate. Mephenytoin hydroxylation is a stereospecific reaction and is confined to the S-enantiomer, which is normally eliminated within hours, allowing the R-enantiomer to accumulate since it can be eliminated only within days or weeks.
W H Theodore et al.
Neurology, 34(8), 1100-1102 (1984-08-01)
We investigated the conversion of mephenytoin to nirvanol in five patients with uncontrolled complex partial seizures. After a 50-mg single oral dose, mean peak mephenytoin level was 0.48 microgram/ml and nirvanol 0.37 microgram/ml. After 400 mg, peak mephenytoin level was
S H Akrawi et al.
European journal of drug metabolism and pharmacokinetics, 14(4), 269-278 (1989-10-01)
The stereoselective clearances of R- and S-mephenytoin were determined in rats receiving either an intravenous or hepatic portal vein infusion of racemic mephenytoin. The mean +/- SD intravenous clearances of R- and S-mephenytoin were 1630 +/- 250 ml/hr and 630
A Pezeshk et al.
Journal of inorganic biochemistry, 42(4), 267-272 (1991-06-01)
The preparation and spectral properties of copper(II) complexes of two hydantoins are reported. Complexes of the general formula Cu(hyd)2(py)2, where hyd = phenytoin or nirvanol; and py = pyridine were prepared and characterized by infrared and ESR. Spectral data show
B F Bourgeois et al.
Epilepsia, 27(4), 412-418 (1986-07-01)
Stereoselective metabolism has been demonstrated for mephenytoin (MHT), R-MHT being demethylated to the pharmacologically active metabolite 5-phenyl-5-ethylhydantoin (PEH; nirvanol), and S-MHT undergoing aromatic hydroxylation to 4-OH-MHT, with formation of an intermediate arene oxide metabolite. PEH is responsible for the therapeutic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service