N7764
Nicotinic acid mononucleotide
Synonym(s):
Nicotinate mononucleotide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
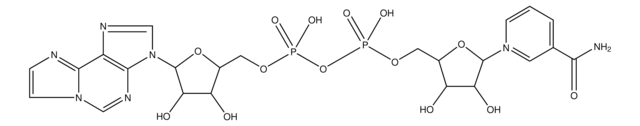
Empirical Formula (Hill Notation):
C11H14NO9P
CAS Number:
Molecular Weight:
335.20
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.79
Recommended Products
biological source
animal
Quality Level
Assay
≥98.0% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
white
storage temp.
−20°C
SMILES string
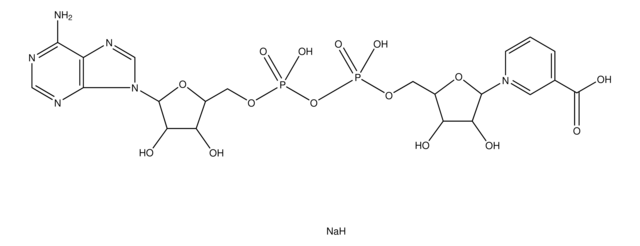
OC1C(O)C(OC1COP(O)(O)=O)[N]2=CC(=CC=C2)C(O)=O
InChI
1S/C11H15NO9P/c13-8-7(5-20-22(17,18)19)21-10(9(8)14)12-3-1-2-6(4-12)11(15)16/h1-4,7-10,13-14H,5H2,(H,15,16)(H2,17,18,19)
InChI key
IUJWGLOJJKFASX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Nicotinic acid mononucleotide (NaMN) is formed from nicotinic acid 5-phosphoribosyl-1-pyrophosphate (PRPP) in the Preiss-Handler pathway in the presence of enzyme phosphoribosyltransferase (NaPRT).
Application
Nicotinic acid mononucleotide has been used as a substrate for NMN/NaMN adenylyltransferase (NMNAT) during nicotinamide adenine dinucleotide (NAD) biosynthesis.
Biochem/physiol Actions
Nicotinic acid mononucleotide (NaMN) may be used to study the relationship between the phosphate-responsive signaling (PHO) pathway and nicotine adenine dinucleotide (NAD(+)) metabolism in yeast. Nicotinate mononucleotide is a substrate for NMN/NaMN adenylyltransferase (NMNAT) and nicotinate mononucleotide adenylyltransferase. NaMN amidation results in the synthesis of nicotinamide mononucleotide(NMN), which is further converted to NAD cofactor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Crystal structure of human nicotinic acid phosphoribosyltransferase
Marletta AS, et al.
FEBS Open Bio, 5, 419-428 (2015)
Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy
Sasaki Y, et al.
Journal of neurosciences in rural practice, 26(33), 8484-8491 (2006)
Determining NAD synthesis in erythrocytes.
V Micheli et al.
Methods in enzymology, 280, 211-221 (1997-01-01)
Shu-Ping Lu et al.
The Journal of biological chemistry, 286(16), 14271-14281 (2011-02-26)
Nicotinamide adenine dinucleotide (NAD(+)) is an essential cofactor involved in various cellular biochemical reactions. To date the signaling pathways that regulate NAD(+) metabolism remain unclear due to the dynamic nature and complexity of the NAD(+) metabolic pathways and the difficulty
Kazuo Yamada et al.
Analytical biochemistry, 352(2), 282-285 (2006-04-01)
We have developed a liquid chromatographic-tandem mass spectrometric method that is sensitive and specific and that simultaneously measures cellular NAD(+) and related compounds. Using this method, NAD(+), NAAD, NMN, NAMN, NAM, NA, ADPR, and 5'AMP were first separated over a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service