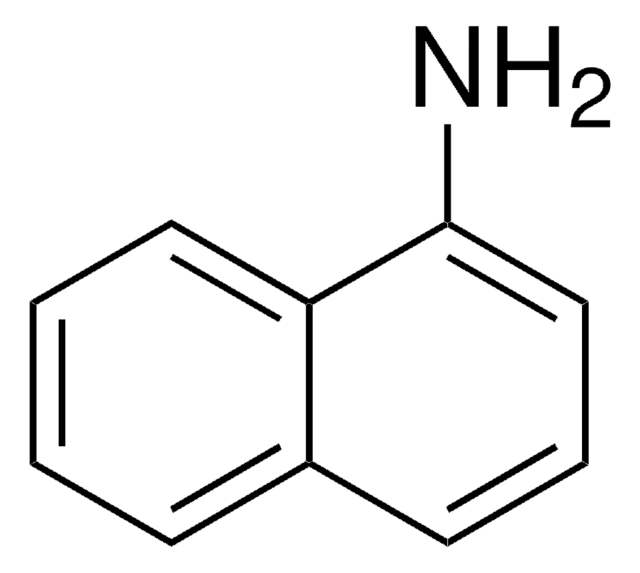
L1635
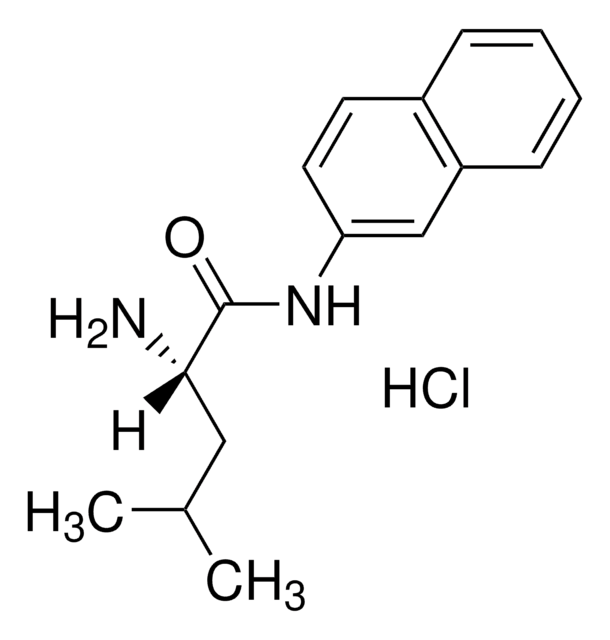
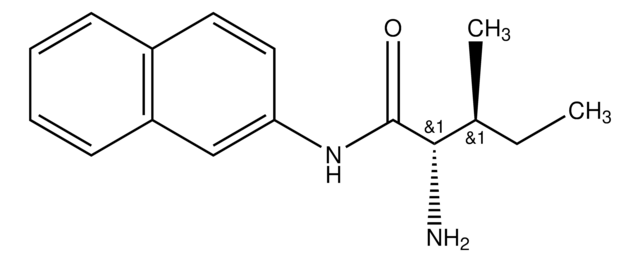
L-Leucine β-naphthylamide
Synonym(s):
L-Leucine-2-naphthylamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H20N2O
CAS Number:
Molecular Weight:
256.34
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.83
Recommended Products
Assay
≥98% (TLC)
Quality Level
form
powder
solubility
H2O: insoluble
storage temp.
−20°C
SMILES string
CC(C)C[C@H](N)C(=O)Nc1ccc2ccccc2c1
InChI
1S/C16H20N2O/c1-11(2)9-15(17)16(19)18-14-8-7-12-5-3-4-6-13(12)10-14/h3-8,10-11,15H,9,17H2,1-2H3,(H,18,19)/t15-/m0/s1
InChI key
JWHURRLUBVMKOT-HNNXBMFYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
L-Leucine β-naphthylamide has been used as a substrate:
- to measure the activity of aminopeptidase in Escherichia coli
- to evaluate the enzyme activity of cathepsin H from rabbit skeletal muscles
- in the proteolytic assay of L-Leucine aminopeptidase
Substrate for leucine aminopeptidase determination in colorimetric and histochemical procedures.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Quality
Very low free β-naphthylamine.
Substrates
Substrate for aminopeptidase M
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Proteolytic activity in the placenta, decidua and postimplantation embryos of the rat.
A Fein et al.
Israel journal of medical sciences, 21(4), 394-396 (1985-04-01)
A M Reisenauer et al.
Science (New York, N.Y.), 227(4682), 70-72 (1985-01-04)
The regulation of amino-oligopeptidase (AOP), an intestinal brush border hydrolase essential for the surface digestion of peptide nutrients, was examined in rats in vivo. Short-term (30-minute) intraintestinal perfusion of a tetrapeptide substrate, Gly-Leu-Gly-Gly, or a synthetic substrate, leucyl-beta-naphthylamide, induced a
T Nishimura et al.
European journal of biochemistry, 137(1-2), 23-27 (1983-12-01)
The mode of action towards oligopeptides and proteins of hydrolase H purified from rabbit skeletal muscle was studied. The presence of protamine or alpha-N-benzoylarginine p-nitroanilide (an endopeptidase substrate) changed both the Km and V values of the enzyme towards Leu-beta-naphthylamide
Aminopeptidase (arylamidase) activity in discrete areas of the rat brain: sex differences.
J M de Gandarias et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 21(5), 285-286 (1989-05-01)
Masanori Matsuishi et al.
The international journal of biochemistry & cell biology, 35(4), 474-485 (2003-02-05)
Rabbit muscle cathepsin H classified as an aminoendopeptidase was purified and its properties were investigated to clarify its contribution to the proteolysis of postmortem muscle. The purification was performed by ammonium sulfate fractionation and successive chromatographies on Sephadex G-75, phosphocelluose
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service