I135
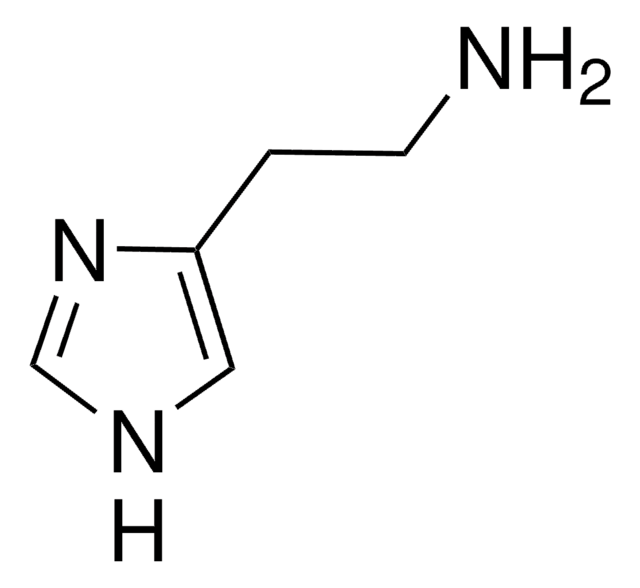
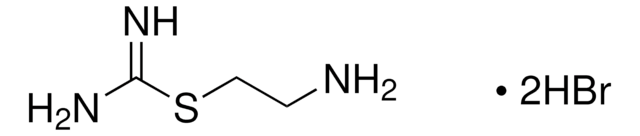
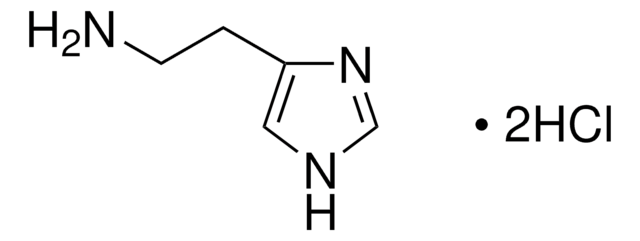
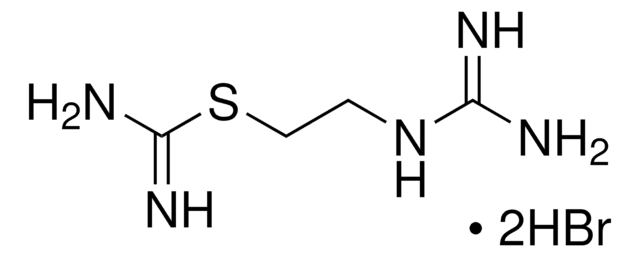
Imetit dihydrobromide
≥98% (HPLC)
Synonym(s):
S-[2-(Imidazol-4-yl)ethyl]isothiourea dihydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10N4S · 2HBr
CAS Number:
Molecular Weight:
332.06
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
H2O: 20 mg/mL, clear
SMILES string
Br[H].Br[H].NC(=N)SCCc1c[nH]cn1
InChI
1S/C6H10N4S.2BrH/c7-6(8)11-2-1-5-3-9-4-10-5;;/h3-4H,1-2H2,(H3,7,8)(H,9,10);2*1H
InChI key
DOBOYMKCRRLTRF-UHFFFAOYSA-N
Gene Information
human ... HRH3(11255)
Biochem/physiol Actions
Potent and selective H3 histamine receptor agonist.
Features and Benefits
This compound is featured on the Histamine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Garbarg et al.
The Journal of pharmacology and experimental therapeutics, 263(1), 304-310 (1992-10-01)
The effects of a new agonist of histamine (HA) H3 receptors, Imetit (S-[2-(4-(imidazolyl)ethyl]isothiourea) were investigated in vitro and in vivo and compared to those of (R)-alpha-methylhistamine [(R)-alpha-MeHA], a prototypic drug. Imetit inhibited the binding of [3H](R-alpha-MeHA to rat brain membranes
Balasubramaniam Annamalai et al.
ACS chemical neuroscience, 11(3), 466-476 (2020-01-10)
Reuptake and clearance of released serotonin (5-HT) are critical in serotonergic neurotransmission. Serotonin transporter (SERT) is mainly responsible for clearing the extracellular 5-HT. Controlled trafficking, phosphorylation, and protein stability have been attributed to robust SERT activity. H3 histamine receptors (H3Rs)
Nayeli Rivera-Ramírez et al.
Neurochemistry international, 101, 38-47 (2016-11-05)
The histamine H3 receptor (H3R) is abundantly expressed in the Central Nervous System where it regulates several functions pre and postsynaptically. H3Rs couple to Gαi/o proteins and trigger or modulate several intracellular signaling pathways, including the cAMP/PKA pathway and the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service