All Photos(1)
About This Item
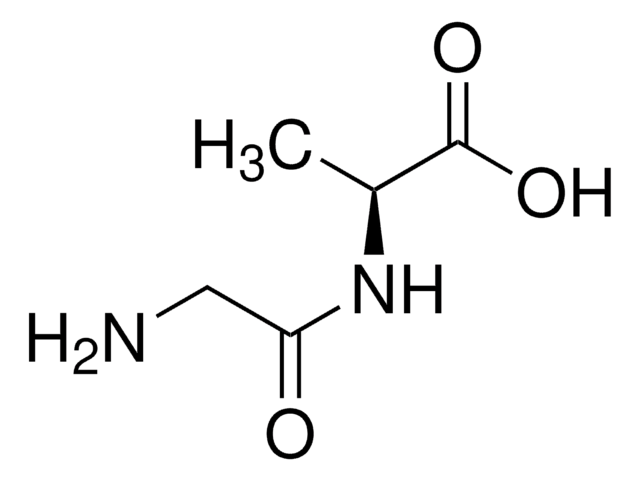
Linear Formula:
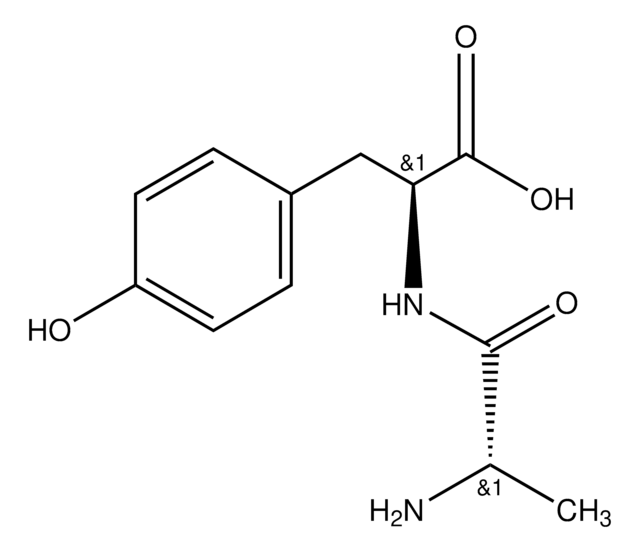
NH2CH2CONHCH(COOH)CH2C6H4OH
CAS Number:
Molecular Weight:
238.24
Beilstein:
2700715
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Gly-Tyr,
Assay
≥98% (TLC)
Quality Level
form
powder
color
white
mp
282 °C
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O
InChI
1S/C11H14N2O4/c12-6-10(15)13-9(11(16)17)5-7-1-3-8(14)4-2-7/h1-4,9,14H,5-6,12H2,(H,13,15)(H,16,17)/t9-/m0/s1
InChI key
XBGGUPMXALFZOT-VIFPVBQESA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Elucidating uptake and metabolic fate of dipeptides in CHO cell cultures using (13)C labeling experiments and kinetic modeling: This study delves into the uptake and metabolic processing of dipeptides such as Gly-Tyr in cell cultures, providing insights into their utilization and role in enhancing biopharmaceutical production processes (Naik et al., 2024).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E C Meng et al.
Proteins, 17(3), 266-278 (1993-11-01)
The biological activities of proteins depend on specific molecular recognition and binding. Computational methods for predicting binding modes can facilitate the discovery and design of ligands and yield information on the factors governing complementarity. The DOCK suite of programs has
L J Wykes et al.
The American journal of physiology, 267(5 Pt 1), E672-E679 (1994-11-01)
Low tyrosine solubility in total parenteral nutrition (TPN) solutions complicates meeting the aromatic amino acid needs of infants. This study compared the effectiveness of two tyrosine precursors to supply the aromatic amino acid needs of TPN-fed neonatal piglets with a
P Stehle et al.
The Journal of nutrition, 126(3), 663-667 (1996-03-01)
Poor solubility hampers the addition of sufficient amounts of free tyrosine to parenteral amino solutions. We investigated the use of a highly soluble synthetic dipeptide, glycyl-L-tyrosine, as a parenteral tyrosine source in 18 male Wistar rats (body weight 180-200 g).
S Albers et al.
Clinical science (London, England : 1979), 76(6), 643-648 (1989-06-01)
1. A commercial amino acid solution supplemented with two synthetic dipeptides, L-alanyl-L-glutamine (Ala-Gln) and glycyl-L-tyrosine (Gly-Tyr), or alternatively with isonitrogenous amounts of free alanine and glycine has been continuously infused over 4 h in six apparently healthy volunteers. 2. The
P Labute et al.
Journal of medicinal chemistry, 44(10), 1483-1490 (2001-05-04)
A method is presented for flexibly aligning small molecules. The method accepts a collection of small molecules with 3D coordinates as input and computes a collection of alignments. Each alignment is given a score, which quantifies the quality of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service