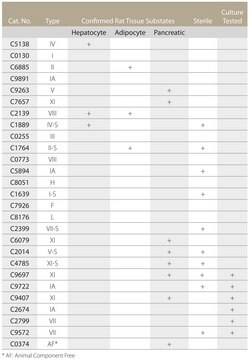
C6885
Collagenase from Clostridium histolyticum
suitable for release of physiologically active rat epididymal adipocytes, Type II, 0.5-5.0 FALGPA units/mg solid, ≥125 CDU/mg solid
Synonym(s):
Clostridiopeptidase A
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
biological source
Clostridium histolyticum
Quality Level
form
powder
specific activity
≥125 CDU/mg solid
0.5-5.0 FALGPA units/mg solid
suitability
suitable for release of physiologically active rat epididymal adipocytes
application(s)
diagnostic assay manufacturing
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Clostridium histolyticum is a pathogenic clostridium that produces collagenase. This is a mixture of enzymes containing collagenase, non-specific proteases and clostripain.
Application
Collagenase may be used:
- for the preparation of arterial tissue for the study of advanced glycosylation end products (AGE)
- for use along with other proteases for the disaggregation of human tumor, mouse kidney, human brain, lung epithelium and many other tissues.
- in liver and kidney perfusion studies, digestion of pancreas, and isolation of nonparenchymal hepatocytes
- for the preparation of viable hepatocytes from rat liver and for the isolation of fat cells from rat adipose tissue
Biochem/physiol Actions
Collagenase is activated by four gram atom calcium per mole enzyme. It is inhibited by ethylene glycol-bis(beta-aminoethyl ether) - N, N, N′,N′-tetraacetic acid, beta-mercaptoethanol, glutathione, thioglycolic acid and 8-hydroxyquinoline.
Collagenase is activated by four gram atom calcium, per mole enzyme. It is inhibited by ethylene glycol-bis(β-aminoethyl ether) - N, N, N′,N′-tetraacetic acid, β-mercaptoethanol, glutathione, thioglycolic acid and 8-hydroxyquinoline.Collagenase enzymes and neutral protease plays an important role in the effective release of cells from tissue. Collagenase recognizes the sequence -R-Pro-8-X-Gly-Pro-R-, where X is most often a neutral amino acid.
Caution
As supplied, this product is stable for one year at -20°C. There is no loss in FALGPA or protease activity in 30 days at 37°C, 50°C and -20°C. Solutions of crude collagenase are stable if frozen quickly in aliquots (at 10 mg/mL) and kept frozen at -20°C. Further freeze-thaw cycles will damage the solution. The product retains 100% activity over 7 hours when held on ice.
Unit Definition
One collagen digestion unit (CDU) liberates peptides from collagen from bovine achilles tendon equivalent in ninhydrin color to 1.0 μmole of leucine in 5 hours at pH 7.4 at 37 °C in the presence of calcium ions. One FALGPA hydrolysis unit hydrolyzes 1.0 μmole of furylacryloyl-Leu-Gly-Pro-Ala per min at 25°C. One Neutral Protease unit hydrolyzes casein to produce color equivalent to 1.0 μmole of tyrosine per 5 hr at pH 7.5 at 37°C. One Clostripain Unit hydrolyzes 1.0 μmole of BAEE per min at pH 7.6 at 25°C in the presence of DTT.
Preparation Note
Solutions are typically prepared at 1-2 mg/mL in TESCA buffer (containing 50 mM TES, 0.36 mM Calcium chloride, pH 7.4 at 37°C.
This product also contains clostripain, nonspecific neutral protease, and tryptic activities.
This product also contains clostripain, nonspecific neutral protease, and tryptic activities.
related product
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C M Posavad et al.
Mucosal immunology, 10(5), 1259-1269 (2017-01-05)
Local mucosal cellular immunity is critical in providing protection from HSV-2. To characterize and quantify HSV-2-reactive mucosal T cells, lymphocytes were isolated from endocervical cytobrush and biopsy specimens from 17 HSV-2-infected women and examined ex vivo for the expression of
Methods in Enzymology, 192, 829-829 (1990)
O Matsushita et al.
Journal of bacteriology, 181(3), 923-933 (1999-01-28)
Clostridium histolyticum collagenase contains a number of different active components. Previously we have shown that colH encodes a 116-kDa collagenase (ColH) and a 98-kDa gelatinase. We purified a different 116-kDa collagenase (ColG) from the culture supernatant and sequenced its gene
Seglen, P.O.
Methods in Cell Biology, 13 (1976)
D. Schomberg and M. Salzmann
Enzymes, 1 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service