C6727
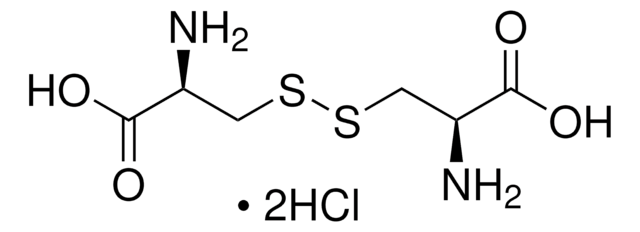
L-Cystine dihydrochloride
≥98.0% dry basis, suitable for cell culture, BioReagent, non-animal source
Synonym(s):
Cystine dihydrochloride
About This Item
Recommended Products
Product Name
L-Cystine dihydrochloride, from non-animal source, BioReagent, suitable for cell culture, ≥98.0% dry basis
biological source
non-animal source
Quality Level
product line
BioReagent
Assay
≥98.0% dry basis
form
powder or crystals
optical activity
[α]20/D -174 to -164 °, c = 2 in 1 M HCl
technique(s)
cell culture | mammalian: suitable
impurities
endotoxin, tested
color
white to off-white
mp
≥211.36 °C
solubility
2 M HCl: 50 mg/mL
cation traces
As: ≤2 ppm
Fe: ≤30 ppm
NH4+: ≤0.03%
heavy metals: ≤10 ppm
functional group
carboxylic acid
thiol
storage temp.
room temp
SMILES string
Cl.Cl.N[C@@H](CSSC[C@H](N)C(O)=O)C(O)=O
InChI
1S/C6H12N2O4S2.2ClH/c7-3(5(9)10)1-13-14-2-4(8)6(11)12;;/h3-4H,1-2,7-8H2,(H,9,10)(H,11,12);2*1H/t3-,4-;;/m0../s1
InChI key
HHGZUQPEIHGQST-RGVONZFCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a supplement to study homopropargylglycine update in metabolomics
- as a supplement in protein synthesis for cell biology and stem cell research
- to study the effect of lipid peroxidation and ferroptosis by regulation of cysteine and GSH levels in biochemical research
Biochem/physiol Actions
Features and Benefits
- High-quality compound suitable for multiple research applications
- Suitable for mammalian cell culture
- Prepared from non-animal source
- Tested for Endotoxins
Caution
Other Notes
Not finding the right product?
Try our Product Selector Tool.
comparable product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service