C3394
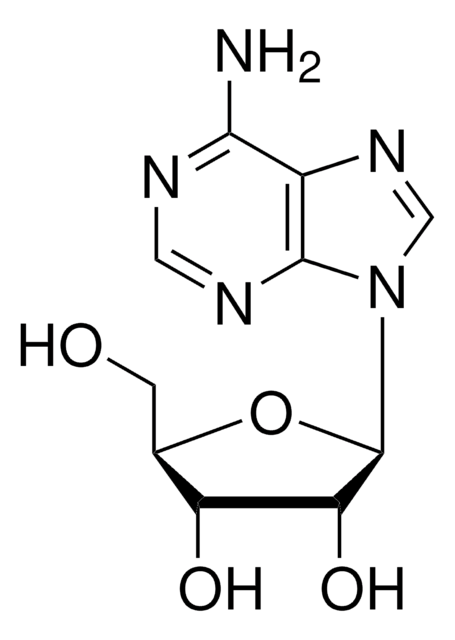
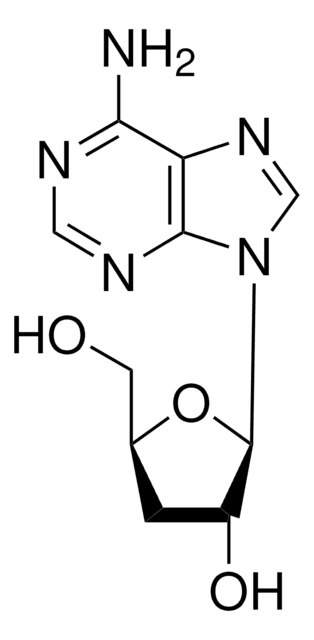
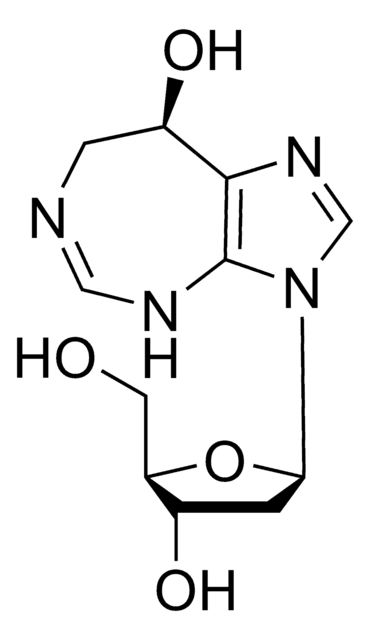
Cordycepin
from Cordyceps militaris, ≥98% (HPLC), powder, adenosine analogue
Synonym(s):
3′-Deoxyadenosine
About This Item
Recommended Products
product name
Cordycepin, from Cordyceps militaris
biological source
Cordyceps militaris
Quality Level
form
powder
antibiotic activity spectrum
fungi
Mode of action
DNA synthesis | interferes
enzyme | inhibits
storage temp.
−20°C
SMILES string
Nc1ncnc2n(cnc12)[C@@H]3O[C@H](CO)C[C@H]3O
InChI
1S/C10H13N5O3/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(17)1-5(2-16)18-10/h3-6,10,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,10+/m0/s1
InChI key
OFEZSBMBBKLLBJ-BAJZRUMYSA-N
Gene Information
rat ... Adora1(29290)
Looking for similar products? Visit Product Comparison Guide
General description
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service