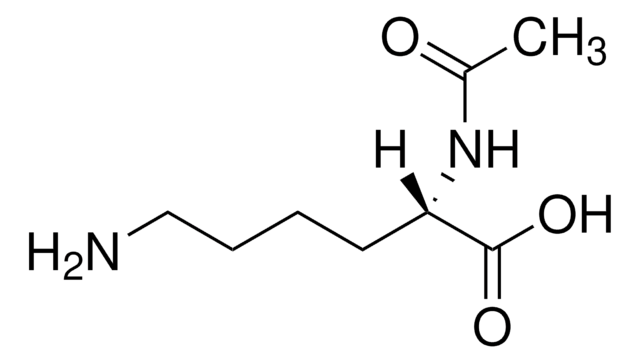
A4021
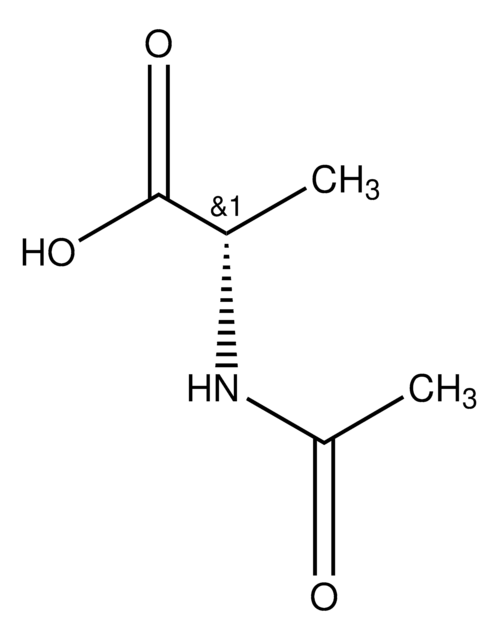
Nε-Acetyl-L-lysine
≥98% (TLC)
Synonym(s):
N6-acetyl-L-lysine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONH(CH2)4CH(NH2)CO2H
CAS Number:
Molecular Weight:
188.22
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Nε-Acetyl-L-lysine,
Assay
≥98% (TLC)
Quality Level
form
powder
concentration
50 mg/mL in 80% acetic acid
color
colorless to white
mp
250 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
CC(=O)NCCCC[C@H](N)C(O)=O
InChI
1S/C8H16N2O3/c1-6(11)10-5-3-2-4-7(9)8(12)13/h7H,2-5,9H2,1H3,(H,10,11)(H,12,13)/t7-/m0/s1
InChI key
DTERQYGMUDWYAZ-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Nε-Acetyl L-α Lysine Improves Activity and Stability of α-Amylase at Acidic Conditions: A Comparative Study with other Osmolytes. This study highlights the use of Nε-Acetyl-ʟ-lysine in enhancing the functional stability and activity of α-amylase under acidic conditions, demonstrating its potential as a valuable additive in industrial enzyme applications (Joghee et al., 2020).
Biochem/physiol Actions
Nε-Acetyl-L-lysine (L-AcK) is an R-chain N-acetylated α amino acid used together with other lysine analogues to differentiate and characterized various aminoacylases and regulator 2 (Sir2) enzymes/sirtuins.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yana Cen et al.
Journal of the American Chemical Society, 132(35), 12286-12298 (2010-08-20)
Sirtuins are protein-modifying enzymes distributed throughout all forms of life. These enzymes bind NAD(+), a universal metabolite, and react it with acetyllysine residues to effect deacetylation of protein side chains. This NAD(+)-dependent deacetylation reaction has been observed for sirtuin enzymes
J A Cohn et al.
Archives of biochemistry and biophysics, 328(1), 158-164 (1996-04-01)
We have previously shown that incubation of the model protein glucose-6-phosphate dehydrogenase (Glu-6-PDH) from the bacterium Leuconostoc mesenteroides with 4-hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, results in the formation of cross-linked protein. HNE-modified protein is resistant to proteolytic
Jarrod B French et al.
Biochemistry, 47(38), 10227-10239 (2008-08-30)
Sirtuins are NAD (+)-dependent enzymes that deacetylate a variety of cellular proteins and in some cases catalyze protein ADP-ribosyl transfer. The catalytic mechanism of deacetylation is proposed to involve an ADPR-peptidylimidate, whereas the mechanism of ADP-ribosyl transfer to proteins is
S L Hazen et al.
The Journal of biological chemistry, 273(9), 4997-5005 (1998-03-28)
We have recently demonstrated that neutrophils oxidize nearly all of the amino acids commonly found in plasma to a corresponding family of aldehydes in high yield. The reaction is mediated by hypochlorous acid (HOCl), the major oxidant generated by the
A Pähler et al.
Chemical research in toxicology, 11(9), 995-1004 (1998-10-07)
Antibodies directed against chemical specific protein modifications are valuable tools to detect and comparatively quantify protein modifications. Both Nepsilon-(dichloroacetyl)-L-lysine and Nepsilon-(trichloroacety)l-L-lysine have been detected as modified amino acids in liver and kidneys of rats treated with perchloroethene (PER) after proteolysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service