A2536
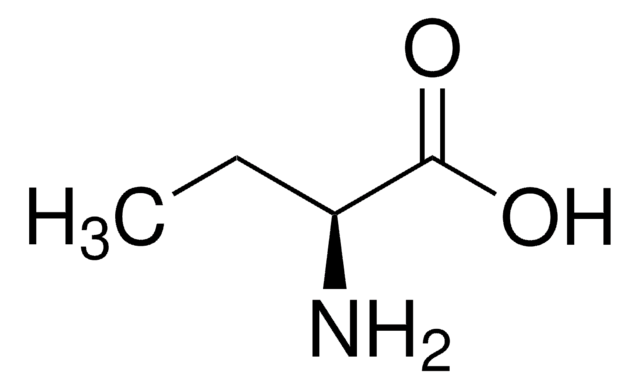
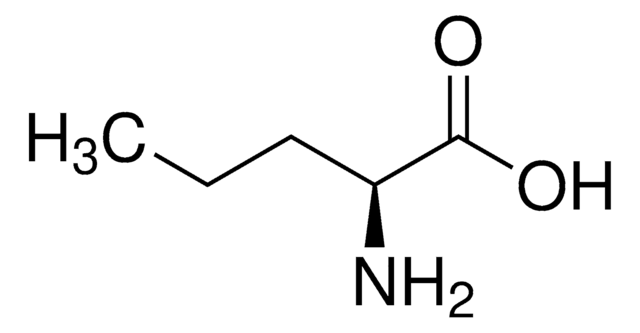
L-2-Aminobutyric acid
BioReagent, suitable for cell culture
Synonym(s):
L-α-Aminobutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H9NO2
CAS Number:
Molecular Weight:
103.12
Beilstein:
1720935
EC Number:
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.75
Recommended Products
biological source
Porcine kidney
microbial
plant
product line
BioReagent
form
powder
technique(s)
cell culture | mammalian: suitable
solubility
water: 50 mg/mL, clear, colorless
shipped in
ambient
storage temp.
room temp
SMILES string
CC[C@H](N)C(O)=O
InChI
1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
InChI key
QWCKQJZIFLGMSD-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-2-Aminobutyric acid is synthesized from L-threonine and L-aspartic acid through a ⍺-transamination reaction. It is an L-alanine analogue with an ethyl side chain.
Biochem/physiol Actions
L-α-Aminobutyric acid (AABA) is an isomer of the non-natural amino acid aminobutyric acid with activity in the γ-glutamyl cycle that regulates glutathione biosynthesis. Recently AABA has been studied as a supplement to in vitro maturation medium (NCSU 23 medium) for culture of oozytes and embryos. This product has been qualified for use in cell culture. AABA is also used as a substitute amino acid for alanine in studies on peptide function.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
l-2-Aminobutyric acid: two fully ordered polymorphs with Z?= 4
Gorbitz CH
Acta Crystallographica Section B, Structural Science, 66(2), 253-259 (2010)
A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADH-regeneration system based on L-leucine dehydrogenase and formate dehydrogenase
Tao R, et al.
Biotechnology Letters, 36(4), 835-841 (2014)
Izumi Kawabata et al.
Nature communications, 3, 722-722 (2012-03-08)
Synaptic remodelling coordinated with dendritic growth is essential for proper development of neural connections. After establishment of synaptic contacts, synaptic junctions are thought to become stationary and provide fixed anchoring points for further dendritic growth. However, the possibility of active
Cornelia Reimmann et al.
Journal of bacteriology, 186(19), 6367-6373 (2004-09-18)
In Pseudomonas aeruginosa, the antibiotic dihydroaeruginoate (Dha) and the siderophore pyochelin are produced from salicylate and cysteine by a thiotemplate mechanism involving the peptide synthetases PchE and PchF. A thioesterase encoded by the pchC gene was found to be necessary
Kechun Zhang et al.
Proceedings of the National Academy of Sciences of the United States of America, 107(14), 6234-6239 (2010-03-25)
The dramatic increase in healthcare cost has become a significant burden to the world. Many patients are denied the accessibility of medication because of the high price of drugs. Total biosynthesis of chiral drug intermediates is an environmentally friendly approach
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service