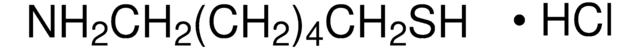30070
Cysteamine
≥98.0% (RT)
Synonym(s):
β-Mercaptoethylamine, 2-Aminoethanethiol, 2-Mercaptoethylamine, Decarboxycysteine, MEA, Thioethanolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH2SH
CAS Number:
Molecular Weight:
77.15
Beilstein:
635649
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (RT)
storage temp.
2-8°C
SMILES string
NCCS
InChI
1S/C2H7NS/c3-1-2-4/h4H,1-3H2
InChI key
UFULAYFCSOUIOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Cysteamine is suitable for use:
- in the preparation of cysteamine modified gold nanoparticles (AuNP)
- in the fabrication of SU-8 microrods, where in, the amine group of cysteamine reacts with the unreacted epoxide rings present on the surface of the particles, thereby opening it and forming a covalent bond
- to enhance in vitro development of porcine oocytes matured and fertilized in vitro
- in a study to demonstrate the depletion effect of cysteamine on cystinotic leucocyte granular fractions of cystine by disulphide interchange
- as a radioprotector
- to administer subcutaneously in rats to study its blocking effect on somatostatin secretion without modifying the pancreatic insulin or glucagon content
- as a scavenger in electrophoretic gels (acetic acid/urea gels)
Biochem/physiol Actions
Cysteamine (β-mercaptoethylamine) depletes cystine from patient′s cells and there by regulates renal glomerular function and increases growth in them. Therefore, cysteamine is considered to be a potential therapeutic for nephropathic cystinosis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Carmichael et al.
Cancer research, 47(4), 943-946 (1987-02-15)
Radiation survival curves were generated for V79 Chinese hamster and two human lung cancer cell lines (NCI-H460 and NCI-H249) with doubling times of 10, 20, and 85 h, respectively, using a standard clonogenic assay, a dye exclusion assay, and a
Automatic microcircuit formation based on gold-coated SU-8 microrods via dielectrophoresis.
Yu-Kun R, et al.
Chinese Physics B, 22 (2013)
C G Grupen et al.
Biology of reproduction, 53(1), 173-178 (1995-07-01)
Most porcine oocytes matured and fertilized in vitro fail to develop normally due to abnormal fertilization. The aim of this study was to determine the effect of cysteamine on pronuclear formation and developmental competence in pig embryos produced in vitro.
R L Sorenson et al.
Diabetes, 32(4), 377-379 (1983-04-01)
Cysteamine (300 mg/kg) administered subcutaneously depletes pancreatic somatostatin to 36% of control levels, but does not alter pancreatic insulin or glucagon content. Although perfusion of pancreata from normal animals with glucose (300 mg/dl) markedly stimulated somatostatin release, pancreata from cysteamine-treated
Cysteamine therapy for children with nephropathic cystinosis
Gahl WA, et al.
The New England Journal of Medicine, 316(16), 971-977 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service