B4380
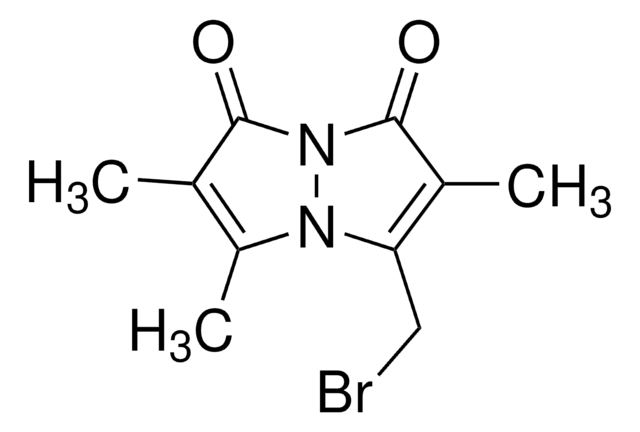
Bromobimane
≥97% purity, powder
Synonym(s):
Monobromobimane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H11BrN2O2
CAS Number:
Molecular Weight:
271.11
Beilstein:
4430959
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
product name
Bromobimane, ≥97% (HPLC)
Quality Level
Assay
≥97% (HPLC)
form
powder
color
yellow
mp
161 °C
solubility
acetonitrile: 20 mg/mL
ε (extinction coefficient)
4.6-5.1 at 396-398 nm in H2O
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
−20°C
SMILES string
CC1=C(C)C(=O)N2N1C(CBr)=C(C)C2=O
InChI
1S/C10H11BrN2O2/c1-5-7(3)12-8(4-11)6(2)10(15)13(12)9(5)14/h4H2,1-3H3
InChI key
AHEWZZJEDQVLOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Bromobimane is also known as monobromobimane. It is also a known probe for thiols and is a fluorescent reagent activated upon a photolysis reaction.
Application
Bromobimane is used for the determination of thiols by the HPLC method. It is suitable as a pre-column derivatization agent for fluorometric determination of 2,3-dimercaptopropane-1-sulfonic acid and other dithiols. Bromobimane has been used as a fluorescent label in studying oligomycin-sensitive ATPase from beef heart mitochondria.
Bromobimane has been used for the quantitative measurement of free hydrogen sulfide in vivo and in vitro. It has been used for the labeling of proteins containing thiol groups.
Bromobimane has been used for the quantitative measurement of free hydrogen sulfide in vivo and in vitro. It has been used for the labeling of proteins containing thiol groups.
Biochem/physiol Actions
Bromobimane in solution reacts with small thiol groups (e.g., GSH) and with reactive protein thiol groups (e.g., hemoglobin). The reaction of Bromobimane with thiols is of second-order and dependent on pH and upon reacting with thiolate, it activates the water-soluble fluorescent product for detection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bromobimane probes for thiols.
E M Kosower et al.
Methods in enzymology, 251, 133-148 (1995-01-01)
N S Kosower et al.
Proceedings of the National Academy of Sciences of the United States of America, 76(7), 3382-3386 (1979-07-01)
The bimane fluorescent labels, monobromobimane, dibromobimane, and monobromotrimethylammoniobimane, are derivatives of syn-9,10-dioxabimane:1,5-diazabicyclo[3.3.0]octa-3,6-diene-2,8-dione. They efficiently label hemoglobin (reactive thiol groups), membrane proteins, and glutathione of normal human red cells under physiological conditions. Monobromobimane and dibromobimane are effective on intact cells while
Differing effects of mechanical dough development and sheeting development methods on aggregated glutenin proteins.
Sutton KH, et al.
Cereal Chem., 80.6, 707-711 (2003)
Xiao Jie Yao et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(23), 9501-9506 (2009-05-28)
G protein-coupled receptors (GPCRs) mediate the majority of physiologic responses to hormones and neurotransmitters. However, many GPCRs exhibit varying degrees of agonist-independent G protein activation. This phenomenon is referred to as basal or constitutive activity. For many of these GPCRs
Edward A Wintner et al.
British journal of pharmacology, 160(4), 941-957 (2010-07-02)
Hydrogen sulphide (H(2)S) is a labile, endogenous metabolite of cysteine, with multiple biological roles. The development of sulphide-based therapies for human diseases will benefit from a reliable method of quantifying H(2)S in blood and tissues. Concentrations of reactive sulphide in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service