39405
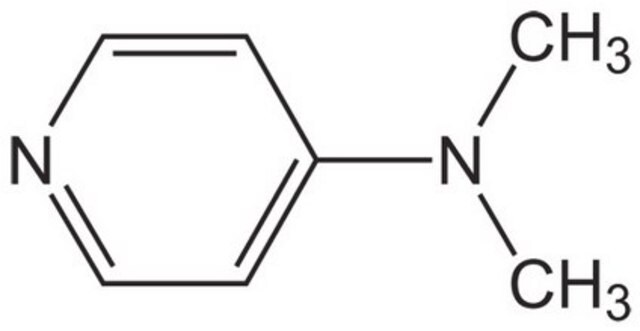
4-(Dimethylamino)pyridine
purum, ≥98.0% (NT)
Synonym(s):
N,N-Dimethylpyridin-4-amine, DMAP
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (NT)
form
crystals
pellets
mp
108-110 °C (lit.)
111-114 °C
solubility
methanol: 0.1 g/mL, clear, colorless to almost colorless
functional group
amine
SMILES string
CN(C)c1ccncc1
InChI
1S/C7H10N2/c1-9(2)7-3-5-8-6-4-7/h3-6H,1-2H3
InChI key
VHYFNPMBLIVWCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a capping agent in the preparation of water-soluble gold nanoparticles.
- As an initiator in the polymerization of epoxy monomers.
- As an auxiliary reagent in the electroless preparation of gold nanotubes applicable in catalysis.
- As a catalyst in the preparation of γ- and δ-lactones via iodolactonization of γ,δ-unsaturated carboxylic acids.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 1
Target Organs
Nervous system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
255.2 °F
Flash Point(C)
124 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service