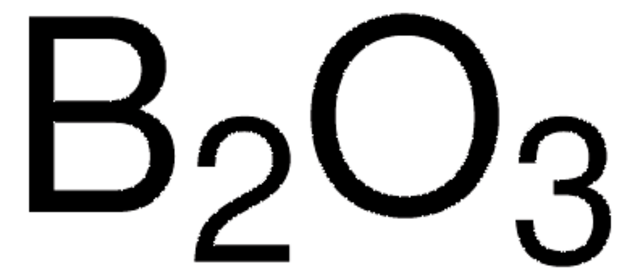15678
Boric anhydride
puriss. p.a., ≥98% (T)
Synonym(s):
Boron trioxide
About This Item
Recommended Products
vapor density
>1 (vs air)
Quality Level
grade
puriss. p.a.
Assay
≥98% (T)
form
solid
loss
≤3% loss on ignition, 900 °C
mp
450 °C (lit.)
density
2.46 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤20 mg/kg
sulfate (SO42-): ≤50 mg/kg
cation traces
Ag: ≤5 mg/kg
Al: ≤50 mg/kg
Ba: ≤5 mg/kg
Bi: ≤5 mg/kg
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤30 mg/kg
Mg: ≤20 mg/kg
Mn: ≤5 mg/kg
Mo: ≤5 mg/kg
Na: ≤200 mg/kg
Ni: ≤5 mg/kg
Pb: ≤30 mg/kg
Sr: ≤5 mg/kg
Tl: ≤10 mg/kg
Zn: ≤5 mg/kg
SMILES string
O=BOB=O
InChI
1S/B2O3/c3-1-5-2-4
InChI key
JKWMSGQKBLHBQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Boron carbon nitride nanosheet (BCN) by thermal condensation reaction with urea and melamine.
- Boron-doped reduced graphene oxide (B-rGO) via a chemical vapor deposition method.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service