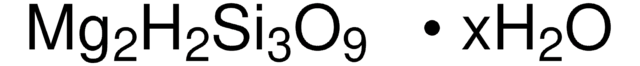All Photos(1)
About This Item
Linear Formula:
Al2O3 · 2SiO2 · 2H2O
CAS Number:
Molecular Weight:
258.16
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
quality
natural
SMILES string
O=[Si]=O.O=[Al]O[Al]=O
InChI
1S/2Al.O2Si.3O/c;;1-3-2;;;
InChI key
HNPSIPDUKPIQMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Kaolinite can be used:
- In the preparation of of polysialate polymers.
- To prepare mullite composites by sintering kaolinite and alumina.
- To synthesize microporous materials such as zeolite Linde Type A (LTA) and kaoline based silicoaluminophosphate (SAPO) molecular sieves.
- In the synthesis of terbium pyridine-picolinate grafted kaoline, as a luminescent hybrid material.
- As a filler and a coating pigment in the paper industry.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New Highly Luminescent Hybrid Materials: Terbium Pyridine? Picolinate Covalently Grafted on Kaolinite.
de Faria E H, et al.
ACS Applied Materials & Interfaces, 3(4), 1311-1318 (2011)
Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers.
Barbosa V F, et al.
International Journal of Inorganic Materials, 2(4), 309-317 (2000)
Preparation of mullite by the reaction sintering of kaolinite and alumina.
Chen C Y, et al.
J. Eur. Ceram. Soc., 20(14-15), 2519-2525 (2000)
Engineered clay products for the paper industry.
Murray H H and Kogel J E
Applied Clay Science, 29(3-4), 199-206 (2005)
Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods.
Rios C A, et al.
Applied Clay Science, 42(3-4), 446-454 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service