MABS157
Anti-O-Linked N-Acetylglucosamine Antibody, clone RL2
clone RL2, from mouse
Synonym(s):
O-Linked N-Acetylglucosamine
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
RL2, monoclonal
species reactivity (predicted by homology)
all
technique(s)
affinity binding assay: suitable
electron microscopy: suitable
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG1κ
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... OGT(8473)
General description
Specificity
Immunogen
Application
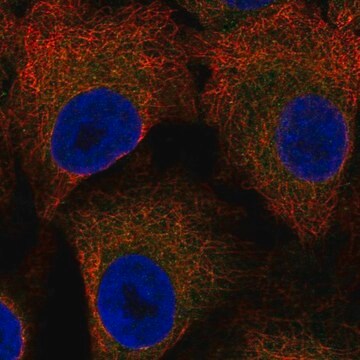
Immunocytochemistry Analysis: A representative lot immunostained nuclear envelopes, but not the nuclear interior, of digitonin-permeabilized HeLa cells. Clone RL2 stained the nuclear interior only among Triton X-100-permeabilized HeLa cells without intact nuclear envelopes (Adam, S.A., et al. (1990). J. Cell Biol. 111(3):807-816).
Affinity Binding Assay: A representative lot was radiolabeled with 125I and studied for its binding characteristics toward isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156).
Electron Microscopy: A representative lot localized the O-GlcNAc immunoreactivity in isolated rat liver nuclear envelopes (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156).
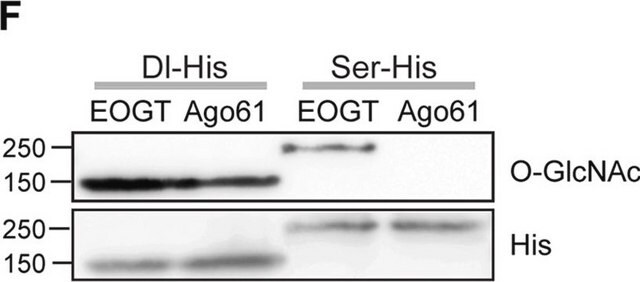
Western Blotting Analysis: A representative lot detected O-GlcNAcylated proteins in rat liver nuclear envelopes preparations (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Immunoprecipitation Analysis: A representative lot immunoprecipitated O-GlcNAcylated proteins from solubilized rat liver nuclear envelopes preparations. Pretreatment of nuclear envelopes preparations with galactosyltrarnsferase prevented the immunoprecipitation of glycoproteins by clone RL2 (Snow, C.M., et al. (1987). J. Cell Biol. 104(5):1143-1156; Holt, G.D., et al. (1987). J. Cell Biol. 104(5):1157-1164).
Quality
Western Blotting Analysis: 1.0 µg/mL of this antibody detected O-Linked N-Acetylglucosamine in 10 µg of HeLa cell lysate.
Target description
Physical form
Other Notes
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service