429700
Leptin, Human, Recombinant, E. coli
Synonym(s):
Leptin, Human, Recombinant, E. coli, rhOB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
sterility
non-sterile
Quality Level
Assay
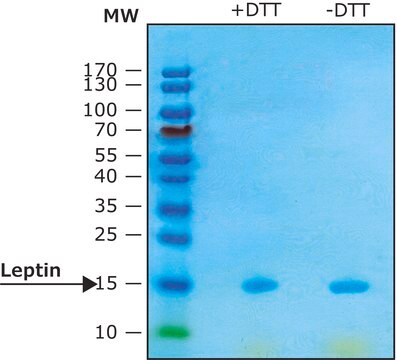
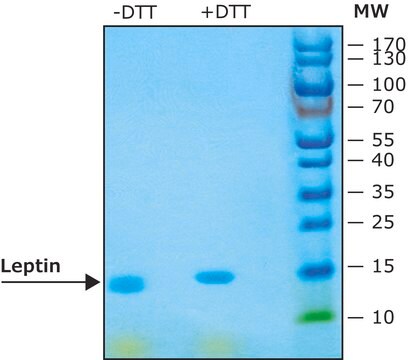
≥97% (SDS-PAGE)
form
lyophilized
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
impurities
≤1.0 EU/μg Endotoxin (EU/μg leptin)
shipped in
ambient
storage temp.
−70°C
General description
Recombinant, human leptin expressed in E. coli. Leptin was originally identified as a protein product of the mouse obese gene. Mice with mutations in the obese gene that block the synthesis of leptin have been found to be obese and diabetic and to have reduced activity, metabolism and body temperature. cDNA clones encoding leptin have been isolated from human, simian, mouse and rat cells. Human leptin shares approximately 84% sequence identity with the mouse protein. Human leptin cDNA encodes a 167 amino acid residue protein with a 21 amino acid signal sequence that is cleaved to yield the 146 amino acid mature protein. The expression of leptin mRNA has been shown to be restricted to adipose tissue.
A high-affinity receptor for leptin (OB-R) with homology to gp130 and the G-CSF receptor was subsequently cloned. The OB-R cytoplasmic domain transduces the leptin signal through the JAK-STAT pathway. Although OB-R mRNA was initially shown to be expressed predominantly in the choroid plexus and in the hypothalamus, more recent data also revealed the expression of this receptor in endothelial cells (Ecs). Furthermore, the angiogenic activity of leptin has been demonstrated both in vitro and in vivo, suggesting a physical mechanism whereby leptin-induced angiogenesis may facilitate increased energy expenditure.
A high-affinity receptor for leptin (OB-R) with homology to gp130 and the G-CSF receptor was subsequently cloned. The OB-R cytoplasmic domain transduces the leptin signal through the JAK-STAT pathway. Although OB-R mRNA was initially shown to be expressed predominantly in the choroid plexus and in the hypothalamus, more recent data also revealed the expression of this receptor in endothelial cells (Ecs). Furthermore, the angiogenic activity of leptin has been demonstrated both in vitro and in vivo, suggesting a physical mechanism whereby leptin-induced angiogenesis may facilitate increased energy expenditure.
Recombinant, human leptin expressed in E. coli. Native leptin is a product of the obese (ob) gene that serves as a ligand for the OB receptor (OB-R). Mice with mutations of the ob gene have been found to be obese and diabetic and to have reduced activity, metabolism, and body temperature. Reported to reduce hepatic glucose production by blocking phosphoenolpyruvate synthesis. Note: Following complete dissolution in 15 mM HCl, add 7.5 mM sterile NaOH and bring the pH to approximately 5.2.
Biochem/physiol Actions
ED₅₀ = 0.4-2 ng/ml as measured by its ability to induce proliferation of leptin-dependent rOB-R transfected murine BAF3 cells
Warning
Toxicity: Standard Handling (A)
Physical form
Lyophilized from a sterile filtered solution in PBS.
Reconstitution
To reconstitute lyophilized leptin, add 15 mM sterile HCl (0.5 ml/1 mg vial or 2.5 ml/5 mg vial) to the vial. After the protein is completely dissolved, add 7.5 mM sterile NaOH (0.3 ml/1mg vial or 1.5 ml/5 mg vial) to bring the pH to ~5.2. Lyophilized samples are stable for at least six months at -70°C. Upon reconstitution, this cytokine can be stored under sterile conditions at 4°C for one month or at -70°C for three months without detectable loss of activity. Avoid repeated freeze/thaw cycles of reconstituted solutions.
Other Notes
Anderwald, C., et al. 2002. Mol. Endocrinol.16, 1612.
Ookuma, M., et al. 1998. Diabetes 47, 219.
Campfield, L.A., et al. 1995. Science 269, 546.
Halaas, J.L., et al. 1995. Science 269, 543.
Pelleymounter, M.A., et al. 1995. Science 269, 540.
Zhang, Y., et al. 1994. Nature372, 425.
Ookuma, M., et al. 1998. Diabetes 47, 219.
Campfield, L.A., et al. 1995. Science 269, 546.
Halaas, J.L., et al. 1995. Science 269, 543.
Pelleymounter, M.A., et al. 1995. Science 269, 540.
Zhang, Y., et al. 1994. Nature372, 425.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service