All Photos(2)
About This Item
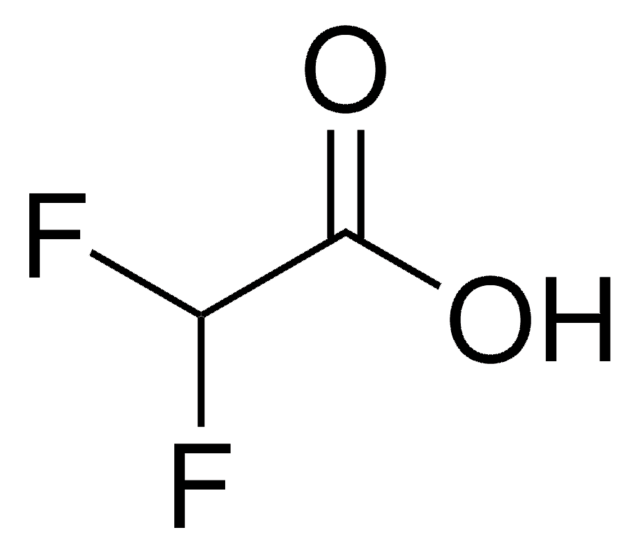
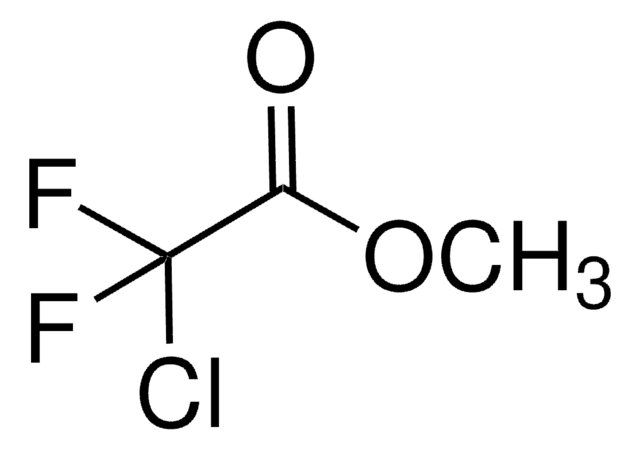
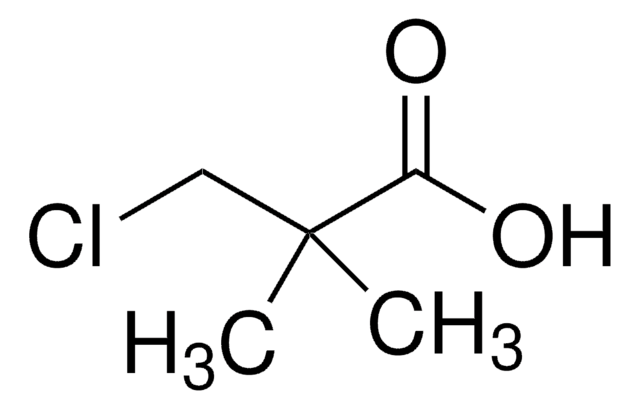
Linear Formula:
F2ClCCOOH
CAS Number:
Molecular Weight:
130.48
Beilstein:
956625
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.355 (lit.)
bp
122 °C (lit.)
mp
20-23 °C (lit.)
density
1.54 g/mL at 25 °C (lit.)
SMILES string
OC(=O)C(F)(F)Cl
InChI
1S/C2HClF2O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
OAWAZQITIZDJRB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J W Harris et al.
Chemical research in toxicology, 4(2), 180-186 (1991-03-01)
1,2-Dichloro-1,1-difluoroethane (HCFC-132b) is a potential substitute for some ozone-depleting chlorofluorocarbons and a model for other 1,1,1,2-tetrahaloethanes under consideration as chlorofluorocarbon substitutes. Male Fischer 344 rats were given 10 mmol/kg HCFC-132b dissolved in corn oil by intraperitoneal injection. An NMR assay
Keith R Solomon et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 2(1), 62-67 (2003-03-28)
Increased UV-B through stratospheric ozone depletion leads to an increased chemical activity in the lower atmosphere (the troposphere). The effect of stratospheric ozone depletion on tropospheric ozone is small (though significant) compared to the ozone generated anthropogenically in areas already
M L Hanson et al.
Environmental toxicology and chemistry, 20(12), 2758-2767 (2002-01-05)
Chlorodifluoroacetic acid (CDFA) is a novel haloacetic acid (HAA) and has been recently documented in aquatic systems. It is a suspected degradation product of the refrigerants 1,1,2-trichloro-1,1-difluoroethane (CFC-113) and 1-chloro-1,1-difluoroethane (HCFC-142b). Haloacetic acids can be phytotoxic, putatively acting through inhibition
M Zdanowska-Fraczek et al.
Solid state nuclear magnetic resonance, 6(2), 141-146 (1996-04-01)
The reorientation of CClF2 groups in N(CH3)4H(ClF2CCOO)2 has been studied using pulsed NQR and NMR techniques. The temperature dependence of both chlorine (35Cl) NQR and fluorine (19F) NMR spin-lattice relaxation has been measured T1Q of chlorine is attributed to the
Janina Kopyra et al.
The Journal of chemical physics, 135(12), 124307-124307 (2011-10-07)
Negative ion formation following resonant electron attachment to the three title molecules is studied by means of a beam experiment with mass spectrometric detection of the anions. All three molecules exhibit a pronounced resonance in the energy range around 1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service