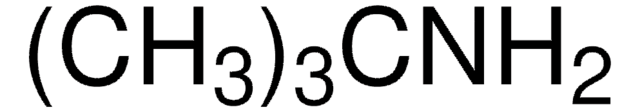C115002
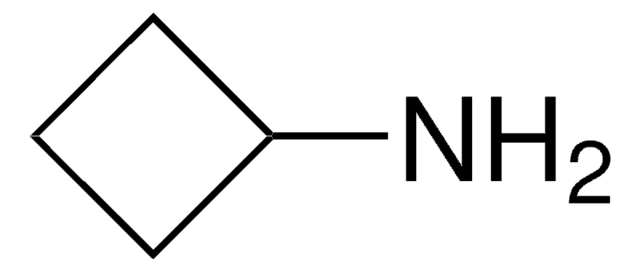
Cyclopentylamine
99%
Synonym(s):
Aminocyclopentane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C5H9NH2
CAS Number:
Molecular Weight:
85.15
Beilstein:
635706
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.450 (lit.)
bp
106-108 °C (lit.)
density
0.863 g/mL at 25 °C (lit.)
SMILES string
NC1CCCC1
InChI
1S/C5H11N/c6-5-3-1-2-4-5/h5H,1-4,6H2
InChI key
NISGSNTVMOOSJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Cycloheptylamineis a versatile compound that has several useful applications in organicsynthesis. Its ability to act as a building block, catalyst, and reagent makesit a valuable tool for the development of new organic compounds. It is alsoused as a reagent for the functionalization of organic molecules.
Application
Cycloheptylamine is a building block for the formation of an ABX3-typed perovskite structure compound [C5H9–NH3][CdCl3].
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
52.7 °F - closed cup
Flash Point(C)
11.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D G Craciunescu et al.
In vivo (Athens, Greece), 1(4), 229-234 (1987-07-01)
Ten new Pt (II) complexes were synthesized and tested as potential antitumor drugs in vitro on KB human tumour cell line, and in vivo against four experimental tumour systems (P388, L1210, ADJ/PC6A and Yoshida sarcoma). The complexes contained two primary
M J Comin et al.
Nucleosides & nucleotides, 18(10), 2219-2231 (2000-01-05)
Purine carbanucleosides built on a 6-oxabicyclo[3.1.0]hexane template were synthesized from readily available 2-cyclopentenone employing a Mitsunobu reaction to incorporate the base onto the carbocyclic ring. Both adenosine and guanosine analogues exhibited moderate antiviral activity.
Maris Vilums et al.
Journal of medicinal chemistry, 56(19), 7706-7714 (2013-09-14)
Preclinical models of inflammatory diseases (e.g., neuropathic pain, rheumatoid arthritis, and multiple sclerosis) have pointed to a critical role of the chemokine receptor 2 (CCR2) and chemokine ligand 2 (CCL2). However, one of the biggest problems of high-affinity inhibitors of
M S Sansom et al.
Protein engineering, 6(1), 65-74 (1993-01-01)
The influenza A M2 protein forms cation-selective ion channels which are blocked by the anti-influenza drug amantadine. A molecular model of the M2 channel is presented in which a bundle of four parallel M2 transbilayer helices surrounds a central ion-permeable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service