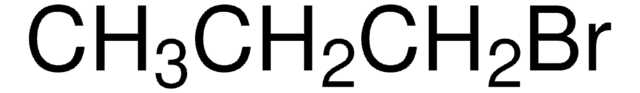All Photos(1)
About This Item
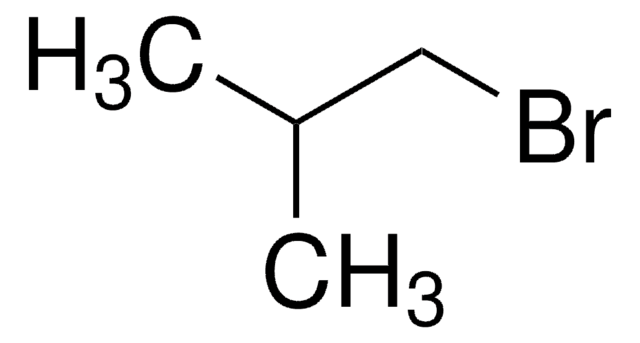
Linear Formula:
CH3CH2CHBrCH3
CAS Number:
Molecular Weight:
137.02
Beilstein:
505949
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.437 (lit.)
bp
91 °C (lit.)
density
1.255 g/mL at 25 °C (lit.)
SMILES string
CCC(C)Br
InChI
1S/C4H9Br/c1-3-4(2)5/h4H,3H2,1-2H3
InChI key
UPSXAPQYNGXVBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Bromobutane is an alkyl halide. Degradation of 2-bromobutane by reductive dehalogenation in the presence of nickel-aluminum alloy in potassium hydroxide solution has been reported. Gas chromatographic determination of 2-bromobutane in mixture of alkyl bromides has been reported. Stereochemistry of dehydrobromination of 2-bromobutane over KOH-silica has been investigated.
Viscosities of binary mixtures of 2-bromobutane and the isomeric forms of butanol have been evaluated at 298.15 and 313.15K. 2,2-dibromobutane, meso-2,3-dibromobutane and dl-2,3-dibromobutane are formed as major photobromination reaction products, during its reaction with molecular bromine. Smaller yields of 1,2-dibromobutane and 2,2,3-tribromobutane are also obtained in this reaction.
Application
2-Bromobutane may be used in the synthesis of series of non-nucleosides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photobromination of (-)-(2R)-2-bromobutane with bromine-81.
Tanner DD, et al.
Journal of the American Chemical Society, 99(8), 2714-2723 (1977)
STEREOCHEMISTRY AND ISOTOPE EFFECT IN THE DEHYDROBROMINATION OF 2-BROMOBUTANE OVER SOLID CATALYSTS.
Misono M and Yoneda Y.
Chemistry Letters (Jpn), 1(7), 551-552 (1972)
Khalid Mohammed Hassan Hilmy
Archiv der Pharmazie, 339(4), 174-181 (2006-04-06)
A series of non-nucleosides 9-47 were synthesized. Compounds 1-4 were reacted with formic acid (85%) to afford compounds 5-8. Then, the latter compounds were reacted with alkyl halides a-f (2-bromopropane, 2-bromobutane, benzyl bromide, benzyl chloromethyl ether, chloromethyl ethyl ether, phenacyl
Selective Reactivity in Gas-Liqiud Chromatography. Determination of 2-Bromobutane and 1-Bromo-2-methylpropane.
Harris WE and McFadden WH.
Analytical Chemistry, 31(1), 114-117 (1959)
Viscosities of binary mixtures of 2-bromobutane and 2-bromo-2-methylpropane with isomeric butanols at 298.15 and 313.15 K.
Artigas H, et al.
International Journal of Thermophysics, 23(6), 1455-1468 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service