A38002
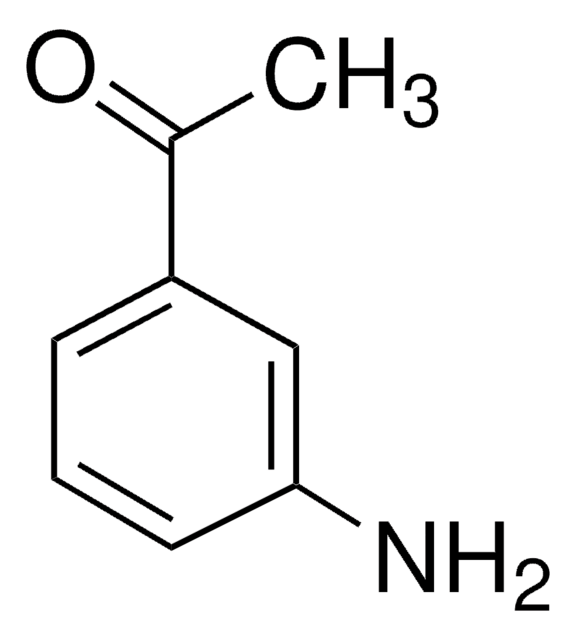
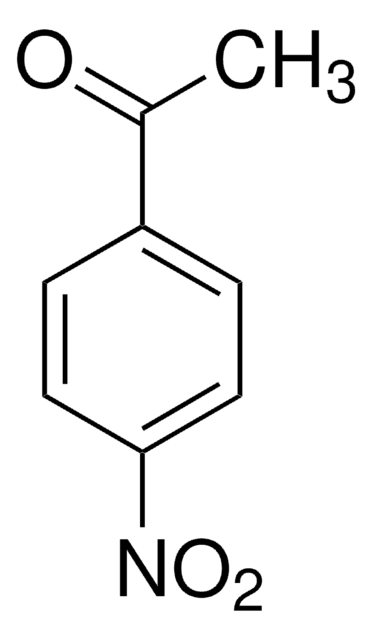
4′-Aminoacetophenone
99%
Synonym(s):
4-Acetylaniline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H4COCH3
CAS Number:
Molecular Weight:
135.16
Beilstein:
471493
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
bp
293 °C (lit.)
mp
103-107 °C (lit.)
SMILES string
CC(=O)c1ccc(N)cc1
InChI
1S/C8H9NO/c1-6(10)7-2-4-8(9)5-3-7/h2-5H,9H2,1H3
InChI key
GPRYKVSEZCQIHD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anne Ly et al.
Organic & biomolecular chemistry, 3(5), 917-923 (2005-02-26)
The most easily oxidized sites in DNA are the guanine bases, and major intermediates produced by the direct effect of ionizing radiation (ionization of the DNA itself) are electron deficient guanine species. By means of a radiation chemical method (gamma-irradiation
Dominik Cincić et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 4), o226-o229 (2008-04-09)
In the title compounds, 4-carboxyanilinium bromide, C(7)H(8)NO(2)(+) x Br(-), (I), and 4-acetylanilinium bromide, C(8)H(10)NO(+) x Br(-), (II), each asymmetric unit contains a discrete cation with a protonated amino group and a halide anion. Both crystal structures are characterized by two-dimensional
O A Aleksintseva et al.
Antibiotiki, 27(7), 493-495 (1982-01-01)
The effect of p-aminobenzoic acid on the biosynthesis of levorin was studied. It was shown that in the presence of exogenic p-aminobenzoic acid the antibiotic activity increased by 11 per cent. The acid added was transformed into p-aminoacetophenone which was
O A Aleksintseva et al.
Antibiotiki, 26(8), 566-570 (1981-08-01)
A method for spectrophotometric determination of p-aminoacetophenone (p-AAP) in the mycelium and fermentation broth filtrates of organisms producing polyenic macrolide antibiotics is described. The level of p-AAP accumulation was studied as applicable to the biosynthesis of levorin, a polyenic antibiotic
O Raatikainen et al.
Journal of chromatography, 585(2), 247-254 (1991-11-01)
A high-performance liquid chromatographic (HPLC) method for the determination of the aromaticity of heptaene polyene antibiotics has been developed. The released aromatic moiety of the heptaene polyenes aureofungin, candicidin, candimycin, hamycin and trichomycin was assayed after alkaline hydrolysis. The presence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service