934437
TissueFab® bioink
(GelHep)MA Vis/ 405nm, low endotoxin
Synonym(s):
3D Bioprinting, Bioink, Controlled Release, GelMA, Gelatin, Gelatin Methacryloyl, HAMA, Heparin, Heparin Methacrylate, Hydrogel
About This Item
Recommended Products
size
10 mL
Quality Level
description
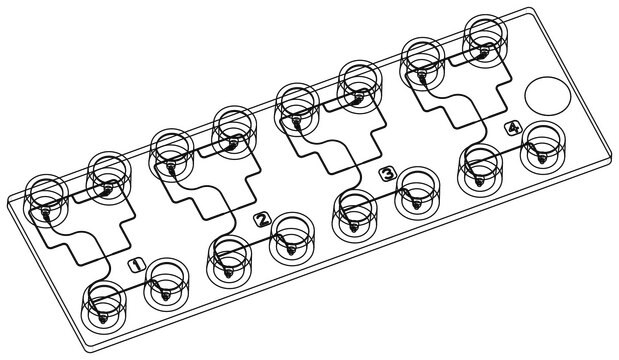
Application: 3D bioprinting
Sterility: 0.2 μm filtered
form
viscous liquid (to gel)
impurities
<5 CFU/g Bioburden (Fungal)
<5 CFU/g Bioburden (total aerobic)
<50 EU/mL Endotoxin
color
pale yellow to colorless
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
About the material:
Heparin is a naturally occurring linear biopolymer and highly sulfated glycosaminoglycan (GAG). Research has demonstrated that heparin can modulate binding extracellular matrix proteins and sequester growth factors and cytokines, making them useful in 3D applications. The methacrylate-functionalization of heparin allows thermal or photochemical crosslinking via covalent conjugation. Heparins exhibit high anionic charge densities to promote large swelling ratios in water. Heparin-based bioinks are used in tissue engineering, 3D bio-printing, and drug delivery applications.
Gelatin methacryloyl (GelMA) is a polymerizable hydrogel material derived from natural extracellular matrix (ECM) components. Due to its low cost, abundance, and retention of natural cell binding motifs, gelatin has become a highly sought material for tissue engineering applications. The addition of photocrosslinkable methacrylamide functional groups in GelMA allows the synthesis of biocompatible, biodegradable, and non-immunogenic hydrogels that are stable in biologically relevant conditions and promote cell adhesion, spreading, and proliferation.
Low Endotoxin, low bioburden: Endotoxins can negatively impact cellular growth, morphology, differentiation, inflammation, and protein expression. The bioburden is the number of contaminated organisms found in a given amount of material.
We test each lot for endotoxins in addition to total bioburden (aerobic and fungal) to minimize unwanted interactions. For more information:
Cell Culture FAQs: Bacterial Endotoxin Contamination
Application
- drug discovery
- in-vitro disease models
- regenerative medicine
- cell-cultured meat
Features and Benefits
- Bioprinting models replicate biology for drug discovery and in vivo applications
- Sterile, low endotoxin
- Batch control offers reproducible models for preclinical toxicology testing and drug screening
- Extended shelf-life & stability
Other Notes
- Ready-to-use formulations eliminate the lengthy bioink formulation development process
- Provide an optimized microenvironment conducive to the growth, proliferation, and maturation of cells
- Validated with widely used cell types (including hMSCs) used in 3D Bioprinting
- Step-by-step protocols developed and tested by internal R&D 3D Bioprinting Scientists
- Compatible with different extrusion-based 3D bioprinter models
Legal Information
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[Ir(df(CF3)ppy)2(4,4′-(OMe)2bpy]BF4 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/294/662/6528b9bf-e008-4b6e-ab53-daa85d4e1dcc/640/6528b9bf-e008-4b6e-ab53-daa85d4e1dcc.png)