907391
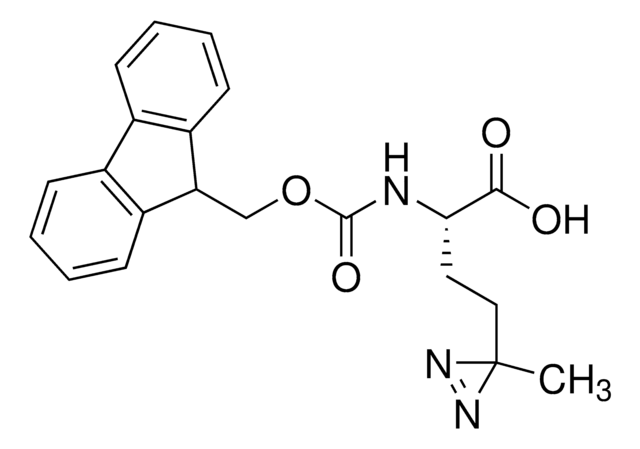
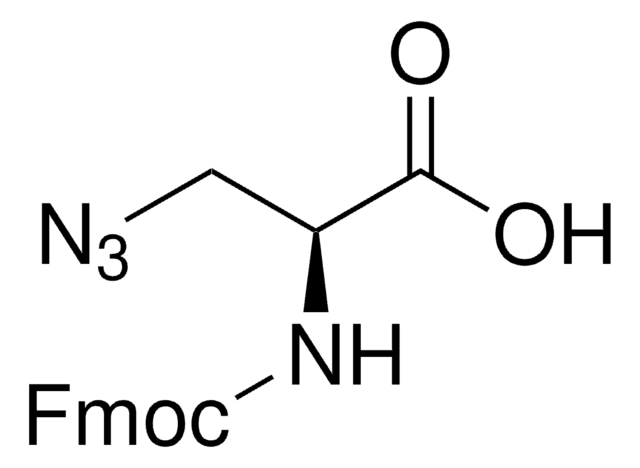
Fmoc-L-photo-leucine
≥98%
Synonym(s):
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-3-(3-methyl-3H-diazirin-3-yl)propanoic acid, (S)-2-(Fmoc-amino)-3-(3H-diazirin-3-yl)butanoic acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Recommended Products
Assay
≥98%
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service